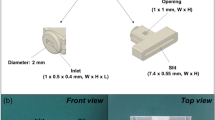
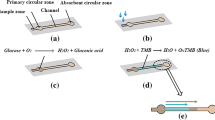
Abstract
Human tears have attracted attention with easy-accessible indicators of healthcare monitoring. Recently self-monitoring of tear glucose in place of blood glucose has been developed with microfluidic paper-based analysis for a non-invasive diagnosis of diabetes. However, most previous works still have many limitations in unstable sample collection, low sensitivity, and insufficient sample volume. Here, we report a colorimetric Schirmer strip with preconcentration for tear glucose detection. A wax barrier on a paper strip enables tear collection of a fixed quantity as well as biocompatible incorporation of tear collection and detection by preventing flow reversal of reagents. Additional solvent was injected on a sampling site after tear collection, which accumulates the dispersed glucose into active site by capillary-driven flow for signal amplification. Glucose concentration was quantitatively detected at 0.1-2 mM by using a simple colorimetric method without any further image processing. The color changes in glucose concentration were clearly distinguishable between normal and diabetic patient-level. The colorimetric paper strip allows straightforward sensing platform for noninvasive diagnosis of diabetes and direct application of paper-based technology to the human body fluids.
Similar content being viewed by others
References
Gelstein, S. et al. Human tears contain a chemosignal. Science 331, 226–230 (2011).
La Belle, J.T. et al. Self-monitoring of tear glucose: the development of a tear based glucose sensor as an alternative to self-monitoring of blood glucose. Chem. Commun. (Camb) 52, 9197–9204 (2016).
Mitsubayashi, K. & Arakawa, T. Cavitas Sensors: Contact Lens Type Sensors & Mouthguard Sensors. Electroanalysis 28, 1170–1187 (2016).
Sen, D.K. & Sarin, G.S. Tear glucose levels in normal people and in diabetic patients. Br. J. Ophthalmol. 64, 693–695 (1980).
Van Haeringen, N.J. Clinical biochemistry of tears. Surv. Ophthalmol. 26, 84–96 (1981).
Lane, J.D., Krumholz, D.M., Sack, R.A. & Morris, C. Tear glucose dynamics in diabetes mellitus. Curr. Eye. Res. 31, 895–901 (2006).
Kim, H.J., Jeong, S. & Noh, H. Quantitative Determination of Tear Glucose Using Paper Based Microfluidic Devices. Korean Chem. Soc. 59, 88–92 (2015).
Zhang, J., Hodge, W., Hutnick, C. & Wang, X. Noninvasive diagnostic devices for diabetes through measuring tear glucose. J. Diabetes. Sci. Technol. 5, 166–172 (2011).
Yan, Q. et al. Measurement of tear glucose levels with amperometric glucose biosensor/capillary tube configuration. Anal. Chem. 83, 8341–8346 (2011).
Peng, B. et al. Evaluation of enzyme-based tear glucose electrochemical sensors over a wide range of blood glucose concentrations. Biosens. Bioelectron. 49, 204–209 (2013).
Liao, Y.T., Yao, H.F., Lingley, A., Parviz, B. & Otis, B.P. A 3-mu W CMOS Glucose Sensor for Wireless Contact- Lens Tear Glucose Monitoring. IEEE J. Solid-state Circuits 47, 335–344 (2012).
Kim, J., Yun, S. & Ounaies, Z. Discovery of cellulose as a smart material. Macromolecules 39, 4202–4206 (2006).
Kim, M.S., Yeon, J.H. & Park, J.K. A microfluidic platform for 3-dimensional cell culture and cell-based assays. Biomed. Microdevices 9, 25–34 (2007).
Han, J. et al. Rapid emergence and mechanisms of resistance by U87 glioblastoma cells to doxorubicin in an in vitro tumor microfluidic ecology. Proc. Natl. Acad. Sci. U S A 113, 14283–14288 (2016).
Martinez, A.W., Phillips, S.T. & Whitesides, G.M. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Natl. Acad. Sci. U S A 105, 19606–19611 (2008).
Shin, J.H., Park, J., Kim, S.H. & Park, J.K. Programmed sample delivery on a pressurized paper. Biomicrofluidics 8, 054121 (2014).
Mu, X., Zhang, L., Chang, S.Y., Cui, W. & Zheng, Z. Multiplex Microfluidic Paper-based Immunoassay for the Diagnosis of Hepatitis C Virus Infection. Anal. Chem. 86, 5338–5344 (2014).
Yu, J.H., Ge, L., Huang, J.D., Wang, S.M. & Ge, S.G. Microfluidic paper-based chemiluminescence biosensor for simultaneous determination of glucose and uric acid. Lab Chip 11, 1286–1291 (2011).
Cheng, C.M. et al. Paper-Based ELISA. Angew. Chem. Int. Ed. 49, 4771–4774 (2010).
Chen, B., Kwong, P. & Gupta, M. Patterned Fluoropolymer Barriers for Containment of Organic Solvents within Paper-Based Microfluidic Devices. Acs Appl. Mater. Interfaces 5, 12701–12707 (2013).
Jungreis, E. Spot test analysis: Clinical, environmental, forensic, and geochemical applications. New York: John Wiley & Sons, 80 (1985).
Jokerst, J.C. et al. Development of a paper-based analytical device for colorimetric detection of select foodborne pathogens. Anal. Chem. 84, 2900–2907 (2012).
Yamada, K., Takaki, S., Komuro, N., Suzuki, K. & Citterio, D. An antibody-free microfluidic paper-based analytical device for the determination of tear fluid lactoferrin by fluorescence sensitization of Tb3+. Analyst 139, 1637–1643 (2014).
Park, M., Jung, H., Jeong, Y. & Jeong, K.H. Plasmonic Schirmer Strip for Human Tear-Based Gouty Arthritis Diagnosis Using Surface-Enhanced Raman Scattering. ACS Nano 11, 438–443 (2017).
Hwang, J., Lee, S. & Choo, J. Application of a SERSbased lateral flow immunoassay strip for the rapid and sensitive detection of staphylococcal enterotoxin B. Nanoscale 8, 11418–11425 (2016).
Park, S.G. et al. Nanoplasmonic Biopatch for in vivo Surface Enhanced Raman Spectroscopy. BioChip J. 8, 289–294 (2014).
Nery, E.W. & Kubota, L.T. Evaluation of enzyme immobilization methods for paper-based devices- A glucose oxidase study. J. Pharm. Biomed. Anal. 117, 551–559 (2016).
Khan, M.S., Li, X., Shen, W. & Garnier, G. Thermal stability of bioactive enzymatic papers. Colloid Surface B 75, 239–246 (2010).
Carrilho, E., Martinez, A.W. & Whitesides, G.M. Understanding Wax Printing: A Simple Micropatterning Process for Paper-Based Microfluidics. Anal. Chem. 81, 7091–7095 (2009).
Schonhorn, J.E. et al. A device architecture for threedimensional, patterned paper immunoassays. Lab Chip 14, 4653–4658 (2014).
Ohashi, Y., Dogru, M. & Tsubota, K. Laboratory findings in tear fluid analysis. Clin. Chim. Acta 369, 17–28 (2006).
Bron, A.J. Diagnosis of dry eye. Surv. Ophthalmol. 45, S221–S226 (2001).
Curto, V.F. et al. Fast prototyping of paper-based microfluidic devices by contact stamping using indelible ink. Rsc Advances 3, 18811–18816 (2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, BH., Park, M. & Jeong, KH. Colorimetric Schirmer strip for tear glucose detection. BioChip J 11, 294–299 (2017). https://doi.org/10.1007/s13206-017-1405-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-017-1405-7