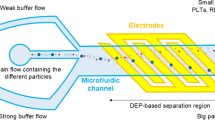
Abstract
Detecting rare cells, such as circulating tumor cells (CTCs), circulating fetal cells, and stem cells, is vital during medical diagnostics and characterization. During carcinogenesis, cancer cells detach from the primary tumor into the blood stream, becoming CTCs. Typical rare cell samples are considered any sample that contains less than 1000 target cells per milliliter. The volumes of microfluidic devices typically range from several microliters to nanoliters; this is excessively small for experimenting using lowconcentration samples. This study involved isolating cancerous cells in an open-top chamber with sub-milliliter volumes (0.1 mL) of blood samples by using a lysis buffer solution for red blood cells (RBCs), as well as concentrating cells employing the dielectrophoretic force generated using stepping electric fields, which were produced using a handheld electric module that comprised a voltage-frequency converter and an operational amplifier. To increase the sample volume, an open-top chamber was fabricated on and bonded to a glass substrate by using circular microelectrodes. The concentrations of cancer cells and RBCs were adjusted to 500 cells/mL and 4×105 cell/mL, respectively, for the experiments. To reduce the interference of blood cells during detection and isolate CTCs, the RBCs in the sample were lysed in a lysis buffer solution before the proposed chip was used to dielectrophoretically manipulate the rare cancerous cells. The findings indicated that the lysis buffer lysed the erythrocytes and the survivability levels of the cancerous cells (HeLa and MCF-7) remained high in the lysis buffer. The positive dielectrophoretic cancerous cells were guided based on the direction of the stepping electric field because of movement in the high-electric-field region; hence, the cancerous cells concentrated and collected at the central electrode.
Similar content being viewed by others
References
Paterlini-Brechot, P. & Benali, N.L. Circulating tumor cells (CTC) detection: Clinical impact and future directions. Cancer Lett. 253, 180–204 (2007).
Mostert, B., Sleijfer, S., Foekens, J.A. & Gratama, J.W. Circulating tumor cells (CTCs): Detection methods and their clinical relevance in breast cancer. Cancer Treat. Rev. 35, 463–474 (2009).
Cheng, X. et al. Enhancing the performance of a pointof-care CD4+ T-cell counting microchip through monocyte depletion for HIV/AIDS diagnostics. Lab Chip 9, 1357–1364 (2009).
Yu, M. et al. Circulating tumor cells: approaches to isolation and characterization. J. Cell Biol. 192, 373–382 (2011).
Dharmasiri, U., Witek, M.A., Adams, A.A. & Soper, S.A. Microsystems for the capture of low-abundance cells. Annu. Rev. Anal. Chem. 3, 409–431 (2010).
Vona, G. et al. Isolation by Size of Epithelial Tumor Cells: A New Method for the Immunomorphological and Molecular Characterization of Circulating Tumor Cells. Am. J. Pathol. 156, 57–63 (2000).
Jacob, K., Sollier, C. & Jabado, N. Circulating tumor cells: detection, molecular profiling and future prospects. Expert Rev. Proteomic. 4, 741–756 (2007).
Hatanaka, H., Yasukawa, T. & Mizutani, F. Detection of Surface Antigens on Living Cells through Incorporation of Immunorecognition into the Distinct Positioning of Cells with Positive and Negative Dielectrophoresis. Anal. Chem. 83, 7207–7212 (2011).
Alunni-Fabbroni, M. & Sandri, M.T. Circulating tumour cells in clinical practice: Methods of detection and possible characterization. Methods 50, 289–297 (2010).
Fu, A.Y., Chou, H.P., Spence, C., Arnold, F.H. & Quake, S.R. An Integrated Microfabricated Cell Sorter. Anal. Chem. 74, 2451–2457 (2002).
Han, K.H., Han, A. & Frazier, A.B. Microsystems for isolation and electrophysiological analysis of breast cancer cells from blood. Biosens. Bioelectron. 21, 1907–1914 (2006).
Koo, O.K. et al. Targeted Capture of Pathogenic Bacteria Using a Mammalian Cell Receptor Coupled with Dielectrophoresis on a Biochip. Anal. Chem. 81, 3094–3101 (2009).
Du, J.R., Juang, Y.J., Wu, J.T. & Wei, H.H. Long-range and superfast trapping of DNA molecules in an ac electrokinetic funnel. Biomicrofluidics 2, 44103 (2008).
Koklu, M., Park, S., Pillai, S.D. & Beskok, A. Negative dielectrophoretic capture of bacterial spores in food matrices. Biomicrofluidics 4, 034107 (2010).
Yang, F. et al. Dielectrophoretic separation of colorectal cancer cells. Biomicrofluidics 4, 013204–013213 (2010).
Sancho, M., Martinez, G., Munoz, S., Sebastian, J.L. & Pethig, R. Interaction between cells in dielectrophoresis and electrorotation experiments. Biomicrofluidics 4, 022802–022811 (2010).
Pethig, R. Preface to Special Topic: Dielectrophoresis. Biomicrofluidics 4, 022701 (2010).
Pethig, R. Review Article-Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics 4, 022811–022835 (2010).
Alazzam, A., Stiharu, I., Bhat, R. & Meguerditchian, A.N. Interdigitated comb-like electrodes for continuous separation of malignant cells from blood using dielectrophoresis. Electrophoresis 32, 1327–1336 (2011).
Becker, F.F. et al. Separation of human breast-cancer cells from blood by differential dielectric affinity. Proc. Natl. Acad. Sci. USA 92, 860–864 (1995).
Wu, L., Lanry Yung, L.Y. & Lim, K.M. Dielectrophoretic capture voltage spectrum for measurement of dielectric properties and separation of cancer cells. Biomicrofluidics 6, 14113–1411310 (2012).
Gascoyne, P.R.C., Noshari, J., Anderson, T.J. & Becker, F.F. Isolation of rare cells from cell mixtures by dielectrophoresis. Electrophoresis 30, 1388–1398 (2009).
Cheng, J., Sheldon, E.L., Wu, L., Heller, M.J. & O’ Connell, J.P. Isolation of Cultured Cervical Carcinoma Cells Mixed with Peripheral Blood Cells on a Bioelectronic Chip. Anal. Chem. 70, 2321–2326 (1998).
Moon, H.S. et al. Continuous separation of breast cancer cells from blood samples using multi-orifice flow fractionation (MOFF) and dielectrophoresis (DEP). Lab Chip 11, 1118–1125 (2011).
Paguirigan, A.L. & Beebe, D.J. Microfluidics meet cell biology: bridging the gap by validation and application of microscale techniques for cell biological assays. Bioessays 30, 811–821 (2008).
Jen, C.P. & Chang, H.H. A handheld preconcentrator for the rapid collection of cancerous cells using dielectrophoresis generated by circular microelectrodes in stepping electric fields. Biomicrofluidics 5, 034101–034110 (2011).
Jen, C.P., Chang, H.H., Huang, C.T. & Chen, K.H. A microfabricated module for isolating cervical carcinoma cells from peripheral blood utilizing dielectrophoresis in stepping electric fields. Microsystem. Technologies 18, 1887–1896 (2012).
Jones, T.B. Electromechanics of particles. Cambridge University Press New York (1995).
Jen, C.P. & Chen, T.W. Selective trapping of live and dead mammalian cells using insulator-based dielectrophoresis within open-top microstructures. Biomedical. Microdevices 11, 597–607 (2009).
Tong, X., Yang, L., Lang, J.C., Zborowski, M. & Chalmers, J.J. Application of immunomagnetic cell enrichment in combination with RT-PCR for the detection of rare circulating head and neck tumor cells in human peripheral blood. Cytometry. B Clin. Cytom. 72, 310–323 (2007).
Mitsuhashi, A. et al. Detection of epidermal growth factor receptor mRNA in peripheral blood of cervical cancer patients. Gynecol. Oncol. 89, 480–485 (2003).
Chen, W. et al. Nanoroughened Surfaces for Efficient Capture of Circulating Tumor Cells without Using Capture Antibodies. ACS Nano. 7, 566–575 (2013).
Toner, M. & Irimia, D. Blood-on-a-Chip. Annu. Rev. Biomed. Eng. 7, 77–103 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, GH., Huang, CT., Wu, HH. et al. Isolating and concentrating rare cancerous cells in large sample volumes of blood by using dielectrophoresis and stepping electric fields. BioChip J 8, 67–74 (2014). https://doi.org/10.1007/s13206-014-8201-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-014-8201-4