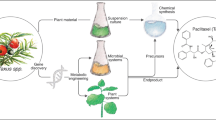
Abstract
Boucerosia diffusa Wight. is an important endangered medicinal plant belonging to the family Asclepiadaceae. In this study, an efficient protocol has been developed for B. diffusa using nodal explants for callus induction and direct organogenesis. The optimal callus induction (83.7%) was observed on 0.6 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D) in Murashige and Skoog medium. The shoot regeneration was observed on different concentrations and combinations of 6-benzylaminopurine (BAP) and 2,4-D using shoot induction (88.5%) was observed on 0.5 mg/L BAP and 0.6 mg/L 2,4-D. Maximum root induction frequency (85.6%) was obtained on 0.6 mg/L α-naphthalene-acetic acid (NAA) and 0.5 mg/L BAP. The fully developed plants were acclimatized (98.86% survival rate) and transferred to natural photoperiod conditions. The phytochemical and pharmacological activity was determined in in vitro-regenerated plants (IRP) and was compared to in vivo wild plants (IWP). The primary and the secondary metabolite contents of bioactive compounds were significantly higher in the methanolic extract of IRP. A comparative antioxidant activity study shows IRP exhibited better scavenging activity. The antidiabetic activity of α- amylase (IC50 − 71.56 ± 15.4 µg/mL) and α-glucosidase (IC50 − 82.94 ± 12.84 µg/mL) inhibitor activity also exhibited maximum in methanolic extract of IRP. Furthermore, chemical composition was analyzed using gas chromatography–mass spectroscopy (GC–MS). Antibacterial activity against human pathogenic bacteria, IRP methanolic extracts showed a maximum zone of inhibition (75 µg/mL) observed against Salmonella typhi (23.5 ± 0.5 mm) compared to the IWP. Molecular docking analysis of B. diffusa inhibition of antidiabetic activity showed better affinity in β-Sitosterol.








Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- BAP:
-
6-benzylaminopurine
- BSA:
-
Bovine serum albumin
- DMSO:
-
Dimethyl sulfoxide
- DPPH⋅:
-
2,2-diphenyl-1-picrylhydrazyl
- EDTA:
-
Ethylene-diamine-tetra-acetic acid
- GC–MS:
-
Gas chromatography–mass spectroscopy
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- IRP:
-
In vitro-regenerated plants
- IWP:
-
In vivo wild plants
- KIN:
-
Kinetin
- MHA:
-
Mueller–Hinton medium,
- MS medium:
-
Murashige and Skoog medium
- NAA:
-
1-naphthaleneacetic acid
- PVPP:
-
Polyvinyl polypyrrolidone
- TDZ:
-
Thidiazuron
- ZOI:
-
Zone of inhibition
References
Adnan M, Jan S, Mussarat S, Tariq A, Begum S, Afroz A, Shinwari ZK (2014) A review on ethnobotany, phytochemistry and pharmacology of plant genus C. aralluma R. Br. J Pharm Pharmacol 66(10):1351–1368. https://doi.org/10.1111/jphp.12265
Ali H, Houghton P, Soumyanath A (2006) α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol 107(3):449–455. https://doi.org/10.1016/j.jep.2006.04.004
Amrati FE-Z, Elmadbouh OHM, Chebaibi M, Soufi B, Conte R, Slighoua M, Saleh A, Al Kamaly O, Drioiche A, Zair T (2022) Evaluation of the toxicity of Caralluma europaea (CE) extracts and their effects on apoptosis and chemoresistance in pancreatic cancer cells. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2022.2135595
Ansari B, Behl T, Pirzada AS, Khan H (2022) Caralluma edulis (Apocynaceae): a comprehensive review on its traditionalu, phytochemical profile and pharmacological effects. Curr Top Med Chem 22(18):1501–1514. https://doi.org/10.2174/1568026622666220527092825
Ashwini S, Anitha R (2017) Antihyperglycemic activity of Caralluma fimbriata: an in vitro approach. Pharmacogn Mag 13(55):S499–S504
Azam A, Ahmed AS, Oves M, Khan MS, Habib SS, Memic A (2012) Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomed 7:6003–6009
Bellamakondi PK, Godavarthi A, Ibrahim M (2018) Caralluma umbellata Haw. protects liver against paracetamol toxicity and inhibits CYP2E1. BioImpacts 8(1):23–30. https://doi.org/10.15171/bi.2018.04
Bouhouche N (2011) Conservation and multiplication of an endangered medicinal plant—Caralluma arabica—using tissue culture. Planta Med 77(12):P49. https://doi.org/10.1055/s-0031-1282303
Bourhia M, Slighoua M, Ibnemoussa S, Bari A, Ullah R, Amaghnouje A, Di Cristo F, El Mzibri M, Calarco A, Benbacer L (2020) Phytochemical study on antioxidant and antiproliferative activities of Moroccan Caralluma europaea extract and its bioactive compound classes. Evid Based Complement Altern Med 2020:1–9. https://doi.org/10.1155/2020/8409718
Braca A, De Tommasi N, Di Bari L, Pizza C, Politi M, Morelli I (2001) Antioxidant principles from Bauhinia tarapotensis. J Nat Prod 64(7):892–895. https://doi.org/10.1021/np0100845
Chandran R, Sajeesh T, Parimelazhagan T (2014) Total phenolic content, anti-radical property and HPLC profiles of Boucerosia diffusa (Wight) NE Br. J Biol Act Prod Nat 4(3):188–195. https://doi.org/10.1080/22311866.2014.933082
Dey P, Kundu A, Kumar A, Gupta M, Lee BM, Bhakta T, Dash S, Kim HS (2020) Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). Recent advances in natural products analysis. Elsevier, Amsterdam, pp 505–567. https://doi.org/10.1016/B978-0-12-816455-6.00015-9
Dinis TC, Madeira VM, Almeida LM (1994) Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 315(1):161–169. https://doi.org/10.1006/abbi.1994.1485
Hodge J (1962) Determination of reducing sugars and carbohydrates. Methods Carbohydr Chem 1:380–394
Janani K, Vignesh A, Veerakumari KP, Vasanth K, Rakkiyappan R (2023) Identifying native endemic plant species in Nilgiris using the interval type 2 q-rung orthopair fuzzy Bonferroni mean operator. Comput Appl Math 42(1):55. https://doi.org/10.1007/s40314-023-02189-x
Kalimuthu K, Kalaiyarasi K, Sasikala T (2011) GC-MS analysis of Boucerosia diffusa (Wight) NE Br. Asian J Plant Sci 3 (4):130–133. https://hal.archives-ouvertes.fr/hal-03694885
Kalimuthu K, Kalaiyarasi K, Prabakaran R, Sasikala T (2014) In vitro propagation of Boucerosia diffusa (Wight) NE Br. Br Biotechnol J 4(2):164–172
Karthik P, Samydurai P, Subbaiyan B, Thangapandian V, Binu T (2013) In vitro propagation of a rare succulent medicinal plant Boucerosia diffusa (Wight) NE Br. Res Plant Biol 3(1):8–17
Kaur G, Rathore T, Rao SR, Shekhawat N (1992) In vitro micropropagation of Caralluma edulis (Edgew.) Benth. & Hook. F.—a rare edible plant species of Indian desert. Indian J Plant Genet Resour 5(1):51–56
Kim G-N, Shin J-G, Jang H-D (2009) Antioxidant and antidiabetic activity of Dangyuja (Citrus grandis Osbeck) extract treated with Aspergillus saitoi. Food Chem 117(1):35–41. https://doi.org/10.1016/j.foodchem.2009.03.072
Kulkarni A, Mute Vaishali S, Dhamane Suchita P, Gadekar A (2012) Evaluation of antibacterial activity of Caralluma adscendens Roxb stem. Int Res J Pharm 3(9):269–270
Kumar KP, George S, Sreedhar S, Balachandran I (2013) Caralluma diffusa (Wight) NE Br. (Apocynaceae)—a new distribution record for Kerala from Chinnar Wild Life Sanctuary, India. Indian for 139(5):425–428
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Makkar HP (2003) Quantification of tannins in tree and shrub foliage: a laboratory manual. Springer, Dordrecht
Malladi S, Ratnakaram VN, Suresh Babu K, Pullaiah T (2017) Evaluation of in vitro antibacterial activity of Caralluma lasiantha for scientific validation of Indian traditional medicine. Cogent Chem 3(1):1374821. https://doi.org/10.1080/23312009.2017.1374821
Malladi S, Ratnakaram VN, Babu KS, Sreenivasulu M (2018) Pharmacological review of Caralluma r. br: a potential herbal genus. Asian J Pharm 12(4):S1146
Mariadoss AVA, Park S, Saravanakumar K, Sathiyaseelan A, Wang MH (2023) Phytochemical profiling, in vitro antioxidants, and antidiabetic efficacy of ethyl acetate fraction of Lespedeza cuneata on streptozotocin-induced diabetic rats. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-023-26412-8
Moore S, Stein WH (1948) Photometric ninhydrin method for use in the chromatography of amino acids. J Biol Chem 176(1):367–388
Mopuri R, Dowlathabad MR, Kommidi DR, Erukainure OL, Rao AA, Rao GV, Islam MS (2022) Anti-diabetic and anti-obesity activity of Caralluma adscendens var. gracilis and Caralluma pauciflora. Res Sq. https://doi.org/10.21203/rs.3.rs-1952085/v1
Motwani DN, Vignesh A, Raja K, Selvakumar S, Vasanth K (2023) Exploration of phytochemicals and probing potential effects of Priva cordifolia active extract on PACAP 38 and its nociceptor in the human trigeminovascular system. 3 Biotech 13(2):39. https://doi.org/10.1007/s13205-023-03462-w
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Ouassou H, Zahidi T, Bouknana S, Bouhrim M, Mekhfi H, Ziyyat A, Aziz M, Bnouham M (2018) Inhibition of α-glucosidase, intestinal glucose absorption, and antidiabetic properties by Caralluma europaea. Evid Based Complement Altern Med 2018:1–8. https://doi.org/10.1155/2018/9589472
Oyaizu M (1988) Antioxidative activities of browning products of glucosamine fractionated by organic solvent and thin-layer chromatography. Nippon Shokuhin Kogyo Gakkaishi 35(11):771–775. https://doi.org/10.3136/nskkk1962.35.11_771
Padwal A, Varpe S, Waman M (2016) Phytochemical and nutritional analysis of Caralluma fimbriata L. Int J Res Biosci Agric Technol 4(1):193–195
Parthipan M, Rajendran A (2014) Extended distribution of Boucerosia diffusa (Wight) NE Br. (Asclepiadaceae) in Tamil Nadu, India. Zoos’ Print 29(9):23–25
Pavan KB, Ashok G, Mohammed I, Ramachandra NM, Rashmi KP (2015) Pharmacognostic evaluation of selected species of Caralluma genus. J Phytopharmacol 4(1):34–40
Prabu M, Sanydurai P, Subbaiyan B, Thangapandian V (2013) Phytochemical constituentsand gas chromatography-Mass spectrometry analysis of Boucerosia diffusa, (Wight) NE BR. Aerial part. Int J Pharm Pharm Sci 5(3):602–605
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269(2):337–434. https://doi.org/10.1006/abio.1999.4019
Raja K, Vignesh A, Lavanya P, Ravi M, Selvakumar S, Vasanth K (2023) Organosulfur compound identified from Striga angustifolia (D. Don) CJ Saldanha inhibits lung cancer growth and induces apoptosis via p53/mTOR signaling pathway. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-023-04467-0
Ramachandran V, Thomas B, Sofiya C, Sasi R (2011) Rediscovery of an endemic plant Boucerosia diffuse (Wight) NE Br. (Asclepiadaceae) from Coimbatore District, Tamil Nadu, India, after 160 years. J Threat Taxa 3(3):1622–1623. https://doi.org/10.11609/JoTT.o2459.1622-3
Reddy AM, Babu MS, Reddy SR, Mohabe S, Devi BA (2016) Caralluma diffusa—a new distributional record for Andhra Pradesh. EPTRI-ENVIS Newsl Eastern Ghats 22(2):1–3
Rehman R, Chaudhary M, Khawar K, Lu G, Mannan A, Zia M (2014) In vitro propagation of Caralluma tuberculata and evaluation of antioxidant potential. Biologia 69(3):341–349. https://doi.org/10.2478/s11756-013-0322-z
Shazmeen N, Nazir M, Riaz N, Saleem M, Tousif MI, Tauseef S, Uddin R, Mukhtar M, Zengin G, Mollica A (2022) In vitro antioxidant and enzyme inhibitory studies, computational analysis and chemodiversity of an emergency food plant Caralluma edulis (Edgew.) Benth. ex Hook. f: a multifunctional approach to provide new ingredients for nutraceuticals and functional foods. Food Biosci 50:102097. https://doi.org/10.1016/j.fbio.2022.102097
Siswadi S, Saragih GS (2021) Phytochemical analysis of bioactive compounds in ethanolic extract of Sterculia quadrifida R. Br. AIP Conf Proc 1(2353):030098. https://doi.org/10.1063/5.0053057
Sodipo O, Akinniyi JA, Ogunbameru J (2000) Studies on certain characteristics of extracts of bark of Pausinystalia johimbe and Pausinystalia macroceras (K Schum) Pierre ex Beille. Glob J Pure Appl Sci 6(1):83–88
Sofowora A, Ogunbodede E, Onayade A (2013) The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med 10(5):210–229. https://doi.org/10.4314/ajtcam.v10i5.2
Ugraiah A, Sreelatha VR, Reddy PK, Rajasekhar K, Rani SS, Karuppusamy S, Pullaiah T (2011) In vitro shoot multiplication and conservation of Caralluma bhupenderiana Sarkaria—an endangered medicinal plant from South India. Afr J Biotechnol 10(46):9328–9336. https://doi.org/10.5897/AJB10.2132
Umdale S, Mahadik R, Otari P, Gore N, Mundada P, Ahire M (2021) Phytochemical composition, and antioxidant potential of Frerea indica Dalz.: a critically endangered, endemic and monotypic genus of the Western Ghats of India. Biocatal Agric Biotechnol 35:102080. https://doi.org/10.1016/j.bcab.2021.102080
Vanitha A, Kalimuthu K, Chinnadurai V, Nisha KJ (2019) Phytochemical screening, FTIR and GC–MS analysis of aqueous extract of Caralluma bicolor—an endangered plant. Asian J Pharm Sci 5(6):1122–1130. https://doi.org/10.31024/ajpp.2019.5.6.7
Venkatesh S, Reddy GD, Reddy BM, Ramesh M, Rao AA (2003) Antihyperglycemic activity of Caralluma attenuata. Fitoterapia 74(3):274–279. https://doi.org/10.1016/S0367-326X(03)00021-2
Vignesh A, Pradeepa Veerakumari K, Selvakumar S, Rakkiyappan R, Vasanth K (2021a) Nutritional assessment, antioxidant, anti-inflammatory and antidiabetic potential of traditionally used wild plant, Berberis tinctoria Lesch. Trends Phytochem Res 5(2):71–92. https://doi.org/10.30495/tpr.2021.1914719.1186
Vignesh A, Selvakumar S, Vasanth K (2021b) Green synthesis and characterization of zinc oxide nanoparticles using Berberis tinctoria Lesch. leaves and fruits extract of multi-biological applications. J Nanomed Res 6:128–147. https://doi.org/10.22034/nmrj.2021.02.005
Vignesh A, Selvakumar S, Vasanth K (2022a) Comparative LC–MS analysis of bioactive compounds, antioxidants and antibacterial activity from leaf and callus extracts of Saraca asoca. Phytomedicine plus 2(1):100167. https://doi.org/10.1016/j.phyplu.2021.100167
Vignesh A, Sivalingam R, Selvakumar S, Vasanth K (2022b) A review on ethnomedicinal and phytopharmacological potential of traditionally wild and endemic plant Berberis tinctoria Lesch. Thai J Pharm Sci 46(2):137–148
Weiskopf SR, Rubenstein MA, Crozier LG, Gaichas S, Griffis R, Halofsky JE, Hyde KJ, Morelli TL, Morisette JT, Muñoz RC (2020) Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci Total Environ 733:137782. https://doi.org/10.1016/j.scitotenv.2020.137782
Yamuna P, Abirami P, Vijayashalini P, Sharmila M (2017) GC-MS analysis of bioactive compounds in the entire plant parts of ethanolic extract of Gomphrena decumbens Jacq. J Med Plants Stud 5(3):31–37
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64(4):555–559. https://doi.org/10.1016/S0308-8146(98)00102-2
Acknowledgements
I would like to thank Dr. M. Parthipan and Dr. I. Kanivalan, Department of Botany, Bharathiar University, Coimbatore, Tamil Nadu, India for plant collection and identification.
Funding
The present study was supported by RUSA 2.0. BCTRC (Ref. No.: IQAC/RUSA 2.0/PA/2021/dated: 08/01/2021) for providing financial support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. AV and TCA contributed equally to material preparation, data collection, analysis, and writing original draft. SJS and SS provided technical support during the initial phases of analysis. KV involved in conceptualization, resources, supervision, funding acquisition, and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest or competing financial interests.
Ethics approval and consent to participate
Not applicable.
Informed consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vignesh, A., Amal, T.C., Janani Sree, S. et al. Conservation linkages of endangered medicinal plant and exploration of phytochemicals, pharmaceutical screening and in silico validation against diabetics using in vivo wild and in vitro regenerated plant Boucerosia diffusa Wight.. 3 Biotech 13, 237 (2023). https://doi.org/10.1007/s13205-023-03645-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03645-5