Abstract
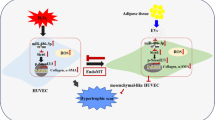
The therapeutic potential of adipose tissue-derived mesenchymal stem cells (ADMSCs) is well studied for use in non-healing wounds. However, concerns on the transplantable cell number requirement, cell expansion, cell viability, retained cell multipotency and the limited cell implantation time for efficient impact hinders cell therapy. Recent literature is much inclined to the superiority of the ADMSCs’ secretome, pre-dominating its paracrine-mediated therapeutic impact. In this context, the possibility of attaining accelerated wound angiogenesis through non-viral mediated enrichment of the ADMSCs secretome with pro-angiogenic growth factors (AGF) seems promising. Accordingly, this study aimed to explore the effect of AGF-enriched ADMSCs secretome for accelerating wound angiogenesis and repair in acute large area full thickness excision rabbit wound model, as adopted from Salgado et al. (Chir Buchar Rom 108:706–710, 1990). Using sub-dermal single-dose injections along the margin of the dorsal wound, native ADMSCs secretome, AGF-enriched ADMSC secretome, allogenic rabbit ADMSCs and a combination of AGF-enriched ADMSC secretome with allogenic rabbit ADMSCs were transplanted independently. Twenty-eight days (28 days) post-transplantation, histopathological analysis was performed to assess the effect. Hematoxylin and eosin (H&E) staining showed enhanced epithelization, notable granulation tissue and collagen fiber deposition in AGF-enriched secretome transplanted groups. This was confirmed by elevated CD31 detection, faster wound closure time and collagen organization. The use of single-dose AGF-enriched ADMSCs’ secretome for therapeutic angiogenesis and wound repair seems to be a promising cell-free therapeutic option. Further investigations using multiple doses on larger animal groups remains to be explored in order to ascertain the comparative potential of AGF-enriched ADMSCs’ secretome.








Similar content being viewed by others
Data availability statement
All data associated with this study is provided in the manuscript.
References
Ajit A, Santhosh Kumar TR, Krishnan LK (2019a) Engineered human adipose-derived stem cells inducing endothelial lineage and angiogenic response. Tissue Eng Part C Methods 25:148–159. https://doi.org/10.1089/ten.tec.2018.0333
Ajit A, Santhosh Kumar TR, Krishnan LK (2019b) Engineered human adipose-derived stem cells inducing endothelial lineage and angiogenic response. Tissue Eng Part C Methods 25:148–159. https://doi.org/10.1089/ten.TEC.2018.0333
Ajit A, Ambika Gopalankutty I (2021) Adipose-derived stem cell secretome as a cell-free product for cutaneous wound healing. 3 Biotech 11:413. https://doi.org/10.1007/s13205-021-02958-7
Billingham R, Medawar P (1951) The technique of free skin grafting in mammals. J Exp Biol 28:385
Choudhry H, Harris AL (2018) Advances in hypoxia-inducible factor biology. Cell Metab 27(2):281–298
de Andrade ALM, Parisi JR, Brassolatti P, Parizotto NA (2017) Alternative animal model for studies of total skin thickness burns. Acta Cir Bras 32:836–842. https://doi.org/10.1590/s0102-865020170100000005
De Becker A, Riet IV (2016) Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy? World J Stem Cells 8:73–87. https://doi.org/10.4252/wjsc.v8.i3.73
Dorsett-Martin WA (2004) Rat models of skin wound healing: a review. Wound Repair Regen off Publ Wound Heal Soc Eur Tissue Repair Soc 12:591–599. https://doi.org/10.1111/j.1067-1927.2004.12601.x
Duscher D, Barrera J, Wong VW et al (2016) Stem cells in wound healing: the future of regenerative medicine? A mini-review. Gerontology 62:216–225. https://doi.org/10.1159/000381877
Ferreira JR, Teixeira GQ, Santos SG et al (2018) Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol. https://doi.org/10.3389/fimmu.2018.02837
Foubert P, Zafra D, Liu M et al (2017) Autologous adipose-derived regenerative cell therapy modulates development of hypertrophic scarring in a red Duroc porcine model. Stem Cell Res Ther 8:261. https://doi.org/10.1186/s13287-017-0704-1
Fujita K, Nishimoto S, Fujiwara T et al (2017) A new rabbit model of impaired wound healing in an X-ray-irradiated field. PLoS ONE 12:e0184534. https://doi.org/10.1371/journal.pone.0184534
Galiano RD, Tepper OM, Pelo CR et al (2004) Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol 164:1935–1947. https://doi.org/10.1016/S0002-9440(10)63754-6
Grada A, Mervis J, Falanga V (2018) Research techniques made simple: animal models of wound healing. J Invest Dermatol 138:2095-2105.e1. https://doi.org/10.1016/j.jid.2018.08.005
Gupta A, Kumar P (2015) Assessment of the histological state of the healing wound. Plast Aesthetic Res 2:239. https://doi.org/10.4103/2347-9264.158862
Hu Y-L, Fu Y-H, Tabata Y, Gao J-Q (2010) Mesenchymal stem cells: a promising targeted-delivery vehicle in cancer gene therapy. J Controlled Release 147:154–162. https://doi.org/10.1016/j.jconrel.2010.05.015
Jackson L, Jones DR, Scotting P, Sottile V (2007) Adult mesenchymal stem cells: differentiation potential and therapeutic applications. J Postgrad Med 53:121–127
Johnson KE, Wilgus TA (2014) Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care 3:647–661. https://doi.org/10.1089/wound.2013.0517
Keeney M, Deveza L, Yang F (2013) Programming stem cells for therapeutic angiogenesis using biodegradable polymeric nanoparticles. J vis Exp JoVE. https://doi.org/10.3791/50736
Laiva AL, O’Brien FJ, Keogh MB (2018) Innovations in gene and growth factor delivery systems for diabetic wound healing. J Tissue Eng Regen Med 12:e296–e312. https://doi.org/10.1002/term.2443
Luli S, Di Paolo D, Perri P et al (2016) A new fluorescence-based optical imaging method to non-invasively monitor hepatic myofibroblasts in vivo. J Hepatol 65:75–83. https://doi.org/10.1016/j.jhep.2016.03.021
Maacha S, Sidahmed H, Jacob S et al (2020) Paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cells Int. https://doi.org/10.1155/2020/4356359
Mitchell R, Mellows B, Sheard J et al (2019) Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res Ther. https://doi.org/10.1186/s13287-019-1213-1
Park KM, Gerecht S (2014) Harnessing developmental processes for vascular engineering and regeneration. Dev Camb Engl 141:2760–2769. https://doi.org/10.1242/dev.102194
Pelizzo G, Avanzini MA, Icaro Cornaglia A et al (2015) Mesenchymal stromal cells for cutaneous wound healing in a rabbit model: pre-clinical study applicable in the pediatric surgical setting. J Transl Med 13:219. https://doi.org/10.1186/s12967-015-0580-3
Ranganath SH, Levy O, Inamdar MS, Karp JM (2012) Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 10:244–258. https://doi.org/10.1016/j.stem.2012.02.005
Ruifrok AC, Johnston DA (2001) Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol 23:291–299
Salgado MI, Petroianu A, Alberti LR et al (2013) Conducted healing to treat large skin wounds. Chir Buchar Rom 108:706–710
Tremolada C, Colombo V, Ventura C (2016) Adipose tissue and mesenchymal stem cells: state of the art and Lipogems® technology development. Curr Stem Cell Rep 2:304–312. https://doi.org/10.1007/s40778-016-0053-5
Wong VW, Sorkin M, Glotzbach JP et al (2011) Surgical approaches to create murine models of human wound healing. J Biomed Biotechnol 2011:969618. https://doi.org/10.1155/2011/969618
Ziadloo A, Burks SR, Gold EM et al (2012) Enhanced homing permeability and retention of bone marrow stromal cells by noninvasive pulsed focused ultrasound. Stem Cells 30:1216–1227. https://doi.org/10.1002/stem.1099
Acknowledgements
The Director, SCTIMST and the Head, BMT Wing is duly acknowledged for extending the necessary facilities to implement this study. The technical support provided by Mr. Prakash R P and Mr. Shankar, Integrated Cancer Research-RGCB to perform electroporation is highly valued. The authors thank Ar. Jobin Joseph Abraham for the technical help in Manuscript Image formatting.
Funding
The study was supported by the Women Scientist Scheme-A (WoS-A) grant awarded to Dr. Amita Ajit, which is funded by the Department of Science and Technology, Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ajit, A., Kumar, T.R.S., Harikrishnan, V.S. et al. Enriched adipose stem cell secretome as an effective therapeutic strategy for in vivo wound repair and angiogenesis. 3 Biotech 13, 83 (2023). https://doi.org/10.1007/s13205-023-03496-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03496-0