Abstract
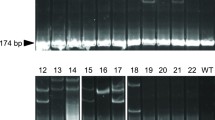
Surrogate broodstock technology can increase the production efficiency of commercially important fishes that are difficult to breed in confinement and aid the propagation and recovery of endangered populations. In this study, we report the application of germ cell (GC) transplantation (GCT) for increasing the numbers of progeny produced by small-bodied ornamental fishes by using sexually mature adult fish as recipients. The GCs isolated from prepubertal male goldfish (Carassius auratus) donors (n = 5) were transplanted through the genital papilla into the gonads of adult common carp (Cyprinus carpio) recipients. The endogenous GCs of the recipient were depleted using busulfan (40 mg/kg body weight [BW]; in five doses at 2-week intervals) and high-temperature (38 °C) treatments. Within 4 months after GCT, the donor GCs recolonised the recipients’ gonads and resumed gametogenesis. The presence of donor-derived gametes was confirmed through polymerase chain reaction–restriction fragment length polymorphism analysis in all the surrogate common carp males and females. Artificial fertilisation and induced spawning between surrogate males and females yielded pure goldfish progeny; the fertilisation and hatching rates were similar to those of the controls. These results suggest that GCT could also be potentially applied in commercial aquaculture, mainly to increase the numbers of progeny obtained from small-bodied fishes those having low gamete counts.











Similar content being viewed by others
Data availability statement
The data that support the findings of this study are available on request from the author.
References
Arregui L, Rathi R, Megee SO, Honaramooz A, Gomendio M, Roldan ER, Dobrinski I (2008) Xenografting of sheep testis tissue and isolated cells as a model for preservation of genetic material from endangered ungulates. Reproduction 136:85–93
Bishai HM, Ishak MM, Labib W (1974) Fecundity of the mirror carp Cyprinus carpio L. at the Serow Fish Farm (Egypt). Aquaculture 4:257–265
Brinster RL (2002) Germ cell transplantation. Science 296:2174–2176
Brinster RL, Zimmermann JW (1994) Spermatogenesis following male germ cell transplantation. Proc Natl Acad Sci USA 91:11298–11302
Brinster CJ, Ryu BY, Avarbock MR, Karagenc L, Brinster RL, Orwig KE (2003) Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol Reprod 69:412–420
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic acids symposium series (Vol. 41, No. 41, 95–98). [London]: Information Retrieval Ltd., c1979-c2000.
Hamra FK, Gatlin J, Chapman KM, Grellhesl DM, Garcia JV, Hammer RE, Garbers DL (2002) Production of transgenic rats by lentiviral transduction of male germ-line stem cells. Proc Natl Acad Sci USA 99:14931–14936
Hermann BP, Sukhwani M, Lin CC, Sheng Y, Tomko J, Rodriguez M, Shuttleworth JJ, McFarland D, Hobbs RM, Pandolfi PP, Schatten GP (2007) Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells 25:2330–2338
Honaramooz A, Yang Y (2010) Recent advances in application of male germ cell transplantation in farm animals. Vet Med Int 2011:657–860
Lacerda SM, Batlouni SR, Costa GM, Segatelli TM, Quirino BR, Queiroz BM, Kalapothakis E, França LR (2010) A new and fast technique to generate offspring after germ cells transplantation in adult fish: the Nile tilapia (Oreochromis niloticus) model. PLoS One 5:e10740
Majhi SK, Hattori RS, Yokota M, Watanabe S, Strüssmann CA (2009) Germ cell transplantation using sexually competent fish: an approach for rapid propagation of endangered and valuable germlines. PLoS One 4:e6132
Majhi SK, Hattori RS, Rahman SM, Strüssmann CA (2014) Surrogate production of eggs and sperm by intrapapillary transplantation of germ cells in cytoablated adult fish. PLoS One 9:e95294
Majhi SK, Rasal AR, Kushwaha B, Raizada S (2017) Heat and chemical treatments in adult Cyprinus carpio (Pisces cypriniformes) rapidly produce sterile gonads. Anim Reprod Sci 183:77–85
Morita T, Kumakura N, Morishima K, Mitsuboshi T, Ishida M, Hara T, Kudo S, Miwa M, Ihara S, Higuchi K, Takeuchi Y (2012) Production of donor-derived offspring by allogeneic transplantation of spermatogonia in the yellowtail (Seriola quinqueradiata). Biol Reprod 86:1–11
Nagano M, Avarbock MR, Brinster RL (1999) Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol Reprod 60:1429–1436
Nagler JJ, Cloud JG, Wheeler PA, Thorgard GH (2001) Testis transplantation in male rainbow trout (Oncorhynchus mykiss). Biol Reprod 64:644–646
Nóbrega RH, Batlouni SR, França LR (2009) An overview of functional and stereological evaluation of spermatogenesis and germ cell transplantation in fish. Fish Physiol Biochem 35:197–206
Ogawa T, Dobrinski I, Brinster RL (1999) Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell 31:461–472
Okutsu T, Suzuki K, Takeuchi Y, Takeuchi T, Yoshizaki G (2006) Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish. Proc Natl Acad Sci USA 103:2725–2729
Okutsu T, Shikina S, Kanno M, Takeuchi Y, Yoshizaki G (2007) Production of trout offspring from triploid salmon parents. Science 317:1517–1517
Ortega-Salas AA, Reyes-Bustamante H (2006) Initial sexual maturity and fecundity of the goldfish Carassius auratus (Perciformes: Cyprynidae) under semi-controlled conditions. Rev Biol Trop 54:1113–1116
Quintana L, Silva A, Berois N, Macadar O (2004) Temperature induces gonadal maturation and affects electrophysiological sexual maturity indicators in Brachyhypopomus pinnicaudatus from a temperate climate. J Exp Biol 207:1843–1853
Saito K, Siegfried KR, Nüsslein-Volhard C, Sakai N (2011) Isolation and cytogenetic characterization of zebrafish meiotic prophase I mutants. Dev Dyn Official Publ Am Assoc Anatomists 240 (7):1779–92
Takeuchi Y, Yoshizaki G, Takeuchi T (2003) Generation of live fry from intraperitoneally transplanted primordial germ cells in rainbow trout. Biol Reprod 69:1142–1149
Takeuchi Y, Yoshizaki G, Takeuchi T (2004) Biotechnology: surrogate broodstock produces salmonids. Nature 430:629
Takeuchi Y, Higuchi K, Yatabe T, Miwa M, Yoshizaki G (2009) Development of spermatogonial cell transplantation in Nibe croaker, Nibea mitsukurii (Perciformes, Sciaenidae). Biol Reprod 81:1055–1063
Vincze T, Posfai J, Roberts RJ (2003) NEBcutter: a program to cleave DNA with restriction enzymes. Nucleic Acids Res 31:3688–3691
Wong TT, Saito T, Crodian J, Collodi P (2011) Zebrafish germline chimeras produced by transplantation of ovarian germ cells into sterile host larvae. Biol Reprod 84:1190–1197
Zeng W, Tang L, Bondareva A, Luo J, Megee SO, Modelski M, Blash S, Melican DT, Destrempes MM, Overton SA, Gavin WG (2012) Non-viral transfection of goat germline stem cells by nucleofection results in production of transgenic sperm after germ cell transplantation. Mol Reprod Dev 79:255–261
Acknowledgements
The author expresses sincere thanks to the Director, ICAR-NBFGR, Lucknow, for providing all necessary help to perform this work. The author also thanks the field supporting staff and research scholars for maintaining the animals.
Funding
This work was supported by a Grant-in-Aid from the ICAR-National Bureau of Fish Genetic Resources (Institutional research Grant#IXX10896).
Author information
Authors and Affiliations
Contributions
SKM conceptualised and designed the experiment, collected data, analysed the data and prepared the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The author declared that no competing interests exist.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Majhi, S.K. Generation of surrogate goldfish Carassius auratus progeny from common carp Cyprinus carpio parents. 3 Biotech 13, 27 (2023). https://doi.org/10.1007/s13205-022-03424-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-022-03424-8