Abstract
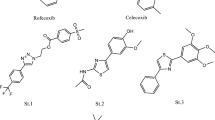
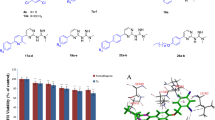
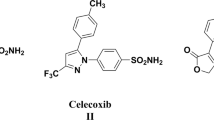
Non-steroidal anti-inflammatory drugs (NSAIDs) are considered one of the most commonly used medications globally. Seventeen isoxazole-containing compounds with various functional groups were evaluated in this work to identify which one was the most potent and which group was most selective toward COX-1 and COX-2 by using an in vitro COX inhibition assay kit. Their cytotoxicity was evaluated on the normal hepatic cell line (LX-2) utilizing the MTS assay. Moreover, these molecules' antibacterial and antifungal activities were evaluated using a microdilution assay against several bacterial and fungal species. In addition, molecular docking studies were conducted to identify the possible binding interactions between these compounds and their biological targets by using the X-ray crystal structure of the human COX enzyme and different proteins of bacterial and fungal strains. At the same time, the QiKProp module was used for ADME-T analysis. The results showed that all evaluated isoxazole derivatives showed moderate to potent activities against COX enzymes. The most potent compound against COX-1 and COX-2 enzymes was A13, with IC50 values of 64 and 13 nM, respectively, and a significant selectivity ratio of 4.63. It was clear that the 3,4-dimethoxy substitution on the first phenyl ring and the Cl atom on the other phenyl pushed the 5-methyl-isoxazole ring toward the secondary binding pocket and created the ideal binding interactions with the COX-2 enzyme in comparison with the other compounds. Compound A8 showed antibacterial and antifungal activities against Pseudomonas aeruginosa, Klebsiella pneumonia, and Candida albicans with MIC values of 2 mg/ml. In fact, this compound showed possible binding interactions with the elastase in P. aeruginosa and KPC-2 carbapenemase in K. pneumonia. Furthermore, for better understanding, molecular dynamics simulations were undertaken to study the change in dynamicity of the protein backbone and ligand after the ligand binds to the protein and to ensure the stability of ligand–protein complexes.








Similar content being viewed by others
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1:19–25
Adasme MF, Linnemann KL, Bolz SN, Kaiser F, Salentin S, Haupt VJ, Schroeder M (2021) PLIP 2021: expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res 49(W1):W530–W534
Assali M, Abualhasan M, Sawaftah H, Hawash M, Mousa A (2020) Synthesis, biological activity, and molecular modeling studies of pyrazole and triazole derivatives as selective COX-2 inhibitors. J Chem. https://doi.org/10.1155/2020/6393428
Bacchi S, Palumbo P, Sponta A, Coppolino MF (2012) Clinical pharmacology of non-steroidal anti-inflammatory drugs: a review. Antiinflamm Antiallergy Agents Med Chem 11(1):52–64. https://doi.org/10.2174/187152312803476255
Badrey MG, Abdel-Aziz HM, Gomha SM, Abdalla MM, Mayhoub AS (2015) Design and synthesis of imidazopyrazolopyridines as novel selective COX-2 inhibitors. Molecules 20(8):15287–15303
Bell EW, Zhang Y (2019) DockRMSD: an open-source tool for atom mapping and RMSD calculation of symmetric molecules through graph isomorphism. J Cheminformatics 11(1):1–9
Bommagani MB, Yerrabelly JR, Chitneni M, Thalari G, Vadiyala NR, Boda SK, Chitneni PR (2021) Synthesis and antibacterial activity of novel cinnoline-isoxazole derivatives. Chem Data Collect 31:100629
Burdan F, Chałas A, Szumiło J (2006) Cyklooksygenaza i prostanoidy–znaczenie biologiczne* cyclooxygenase and prostanoids–biological implications. Postepy Hig Med Dosw 60:129–141
de Groot RA, Nadrchal J (1993) Physics Computing'92: Proceedings of the 4th International Conference. In: Physics Computing'92: Proceedings of the 4th International Conference. Edited by NADRCHAL J ET AL. Published by World Scientific Publishing Co. Pte. Ltd
Eccles SA, Massey A, Raynaud FI, Sharp SY, Box G, Valenti M, Patterson L, de Haven BA, Gowan S, Boxall F (2008) NVP-AUY922: a novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Can Res 68(8):2850–2860
Eid AM, Hawash M, Amer J, Jarrar A, Qadri S, Alnimer I, Sharaf A, Zalmoot R, Hammoudie O, Hameedi S (2021) Synthesis and biological evaluation of novel isoxazole-amide analogues as anticancer and antioxidant agents. BioMed Res Int. https://doi.org/10.1155/2021/6633297
Elokely KM, Doerksen RJ (2013) Docking challenge: protein sampling and molecular docking performance. J Chem Inf Model 53(8):1934–1945
FitzGerald GA, Patrono C (2001) The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med 345(6):433–442
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47(7):1739–1749
Galdino ACM, de Oliveira MP, Ramalho TC, de Castro AA, Branquinha MH, Santos AL (2019) Anti-virulence strategy against the multidrug-resistant bacterial pathogen Pseudomonas aeruginosa: pseudolysin (elastase B) as a potential druggable target. Curr Protein Pept Sci 20(5):471–487
Ghabbour HA, Qabeel MM, Eldehna WM, Al-Dhfyan A, Abdel-Aziz HA (2014) Design, synthesis, and molecular docking of 1-(1-(4-chlorophenyl)-2-(phenylsulfonyl) ethylidene)-2-phenylhydrazine as potent nonazole anticandidal agent. J Chem. https://doi.org/10.1155/2014/154357
Griffen EJ, Dossetter AG, Leach AG, Montague S (2018) Can we accelerate medicinal chemistry by augmenting the chemist with big data and artificial intelligence? Drug Discov Today. https://doi.org/10.1016/j.drudis.2018.03.011
Hargrove TY, Friggeri L, Wawrzak Z, Qi A, Hoekstra WJ, Schotzinger RJ, York JD, Guengerich FP, Lepesheva GI (2017) Structural analyses of Candida albicans sterol 14α-demethylase complexed with azole drugs address the molecular basis of azole-mediated inhibition of fungal sterol biosynthesis. J Biol Chem 292(16):6728–6743
Hawash M, Eid AM, Jaradat N, Abualhasan M, Amer J, Naser Zaid A, Draghmeh S, Daraghmeh D, Daraghmeh H, Shtayeh T, Sawaftah H, Mousa A (2020a) Synthesis and biological evaluation of benzodioxole derivatives as potential anticancer and antioxidant agents. Heterocycl Commun 26(1):157–167. https://doi.org/10.1515/hc-2020-0105
Hawash M, Jaradat N, Hameedi S, Mousa A (2020b) Design, synthesis and biological evaluation of novel benzodioxole derivatives as COX inhibitors and cytotoxic agents. BMC Chem 14(1):1–9
Hawash M, Jaradat N, Abualhasan M, Amer J, Levent S, Issa S, Ibrahim S, Ayaseh A, Shtayeh T, Mousa A (2021a) Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives. Open Chem 19(1):855–863
Hawash M, Jaradat N, Bawwab N, Salem K, Arafat H, Hajyousef Y, Shtayeh T, Sobuh S (2021b) Design, synthesis, and biological evaluation of phenyl-isoxazole-carboxamide derivatives as anticancer agents. Heterocycl Commun 27(1):133–141
Hawash M, Kahraman DC, Cetin-Atalay R, Baytas SN (2021c) Induction of apoptosis in hepatocellular carcinoma cell lines by novel indolylacrylamide derivatives: synthesis and biological evaluation. Chem Biodivers 18(5):e2001037
Hawash M, Kahraman DC, Ergun SG, Cetin-Atalay R, Baytas SN (2021d) Synthesis of novel indole-isoxazole hybrids and evaluation of their cytotoxic activities on hepatocellular carcinoma cell lines. BMC Chem 15(1):1–14
Hawash M, Jaradat N, Abualhasan M, Qneibi M, Rifai H, Saqfelhait T, Shqirat Y, Nazal A, Omarya S, Ibrahim T, Sobuh S, Zarour A, Mousa A (2022) Evaluation of cytotoxic, COX inhibitory, and antimicrobial activities of novel isoxazole-carboxamide derivatives. Lett Drug Des Discov 19:1–1. https://doi.org/10.2174/1570180819666220819151002
Hermann M, Ruschitzka F (2006) Coxibs, non-steroidal anti-inflammatory drugs and cardiovascular risk. Intern Med J 36(5):308–319. https://doi.org/10.1111/j.1445-5994.2006.01056.x
Schrödinger L (2016) Schrödinger suite. Schrödinger, LLC, New York, NY
Jaradat N, Al-lahham S, Abualhasan MN, Bakri A, Zaide H, Hammad J, Hussein F, Issa L, Mousa A, Speih R (2018) Chemical constituents, antioxidant, cyclooxygenase inhibitor, and cytotoxic activities of teucrium pruinosum boiss. Essent Oil Biomed Res Int 18:1–9
Jaradat N, Khasati A, Hawi M, Qadi M, Amer J, Hawash M (2021) In vitro antitumor, antibacterial, and antifungal activities of phenylthio-ethyl benzoate derivatives. Arab J Sci Eng 46(6):5339–5344
Kalle AM, Rizvi A (2011) Inhibition of bacterial multidrug resistance by celecoxib, a cyclooxygenase-2 inhibitor. Antimicrob Agents Chemother 55(1):439–442
Khalil A, Jaradat N, Hawash M, Issa L (2021) In vitro biological evaluation of benzodioxol derivatives as antimicrobial and antioxidant agents. Arab J Sci Eng 46(6):5447–5453
Kumar JD, Zanderigo F, Prabhakaran J, Rubin-Falcone H, Parsey RV, Mann JJ (2018) In vivo evaluation of [11C] TMI, a COX-2 selective PET tracer, in baboons. Bioorg Med Chem Lett 28(23–24):3592–3595
Kumar D, Singh P, Jayaraj A, Kumar V, Kumari K, Patel R (2019) A theoretical model to study the interaction of erythro-noscapines with nsP3 protease of chikungunya virus. ChemistrySelect 4(17):4892–4900
Kumar D, Singh P, Jayaraj A, Kumar V, Kumari K, Chandra R, Ramappa VK (2020) Selective docking of pyranooxazoles against nsP2 of CHIKV eluted through isothermally and non-isothermally MD simulations. ChemistrySelect 5(14):4210–4220
Kumar S, Gupta Y, Zak SE, Upadhyay C, Sharma N, Herbert AS, Durvasula R, Potemkin V, Dye JM, Kempaiah P (2021) A novel compound active against SARS-CoV-2 targeting uridylate-specific endoribonuclease (NendoU/NSP15): in silico and in vitro investigations. RSC Medicinal Chemistry 12(10):1757–1764
Lindorff-Larsen K, Piana S, Palmo K, Maragakis P, Klepeis JL, Dror RO, Shaw DE (2010) Improved side-chain torsion potentials for the amber ff99SB protein force field. Proteins Struct Funct Bioinform 78(8):1950–1958
Linton MF, Fazio S (2008) Cyclooxygenase products and atherosclerosis. Drug Discov Today: Ther Strateg 5(1):25–36
Magpantay HD, Malaluan IN, Manzano JAH, Quimque MT, Pueblos KR, Moor N, Budde S, Bangcaya PS, Lim-Valle D, Dahse H-M (2021) Antibacterial and COX-2 inhibitory tetrahydrobisbenzylisoquinoline alkaloids from the Philippine medicinal plant Phaeanthus ophthalmicus. Plants 10(3):462
Mahboubi Rabbani SMI, Zarghi A (2019) Selective COX-2 inhibitors as anticancer agents: a patent review (2014–2018). Expert Opin Ther Pat 29(6):407–427
Malathi K, Anbarasu A, Ramaiah S (2019) Identification of potential inhibitors for Klebsiella pneumoniae carbapenemase-3: a molecular docking and dynamics study. J Biomol Struct Dyn 37(17):4601–4613
Mao J, Yuan H, Wang Y, Wan B, Pieroni M, Huang Q, Van Breemen RB, Kozikowski AP, Franzblau SG (2009) From serendipity to rational antituberculosis drug discovery of mefloquine-isoxazole carboxylic acid esters. J Med Chem 52(22):6966–6978
Meanwell NA (2018) Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J Med Chem 61(14):5822–5880
Meena MK, Kumar D, Kumari K, Kaushik NK, Kumar RV, Bahadur I, Vodwal L, Singh P (2022) Promising inhibitors of nsp2 of CHIKV using molecular docking and temperature-dependent molecular dynamics simulations. J Biomol Struct Dyn 40(13):5827–5835
Nacak S, Ökçelik B, Ünlü S, Şahin MF, Özkan S, Abbasoğlu U (2005) Synthesis and antimicrobial activity of some new mannich bases of 7-acyl-5-chloro-2-oxo-3H-benzoxazole derivatives. Turk J Pharm Sci 2(1):25–33
Orlando BJ, Lucido MJ, Malkowski MG (2015) The structure of ibuprofen bound to cyclooxygenase-2. J Struct Biol 189(1):62–66
Panda S, Chowdary PR, Jayashree B (2009) Synthesis, antiinflammatory and antibacterial activity of novel indolyl-isoxazoles. Indian J Pharm Sci 71(6):684
Panwar U, Singh SK (2021) Atom-based 3D-QSAR, molecular docking, DFT, and simulation studies of acylhydrazone, hydrazine, and diazene derivatives as IN-LEDGF/p75 inhibitors. Struct Chem 32(1):337–352
Paragi-Vedanthi P, Doble M (2010) Comparison of PGH2 binding site in prostaglandin synthases. BMC Bioinformatics 11(1):1–8
Park JY, Pillinger MH, Abramson SB (2006) Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol 119(3):229–240
Plount Price ML, Jorgensen WL (2000) Analysis of binding affinities for celecoxib analogues with COX-1 and COX-2 from combined docking and Monte Carlo simulations and insight into the COX-2/COX-1 selectivity. J Am Chem Soc 122(39):9455–9466
Rao P, Knaus EE (2008) Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci 11(2):81s–110s. https://doi.org/10.18433/j3t886
Ricardo E, Costa-de-Oliveira S, Silva Dias A, Guerra J, Rodrigues AG, Pina-Vaz C (2009) Ibuprofen reverts antifungal resistance on Candida albicans showing overexpression of CDR genes. FEMS Yeast Res 9(4):618–625
Santos MM, Faria N, Iley J, Coles SJ, Hursthouse MB, Martins ML, Moreira R (2010) Reaction of naphthoquinones with substituted nitromethanes. Facile synthesis and antifungal activity of naphtho [2, 3-d] isoxazole-4, 9-diones. Bioorganic Med Chem Lett 20(1):193–195
Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Isakson P (1997) Distribution of COX-1 and COX-2 in normal and inflamed tissues. Eicosanoids and other bioactive lipids in cancer, inflammation, and radiation injury 2. Springer, pp 167–170
Sharma S, Kumar-M P, Singh S, Sohal A, Yadav R, Gupta N (2021) Discovery of dual inhibitors of KPC-3 and KPC-15 of Klebsiella pneumoniae–an in-silico molecular docking and dynamics study. bioRxiv. https://doi.org/10.1101/2019.12.23.886671
Smith WL, Urade Y, Jakobsson P-J (2011) Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem Rev 111(10):5821–5865
Thangamani S, Younis W, Seleem MN (2015) Repurposing celecoxib as a topical antimicrobial agent. Front Microbiol 6:750
Van Aalten DM, Bywater R, Findlay JB, Hendlich M, Hooft RW, Vriend G (1996) PRODRG, a program for generating molecular topologies and unique molecular descriptors from coordinates of small molecules. J Comput Aided Mol Des 10(3):255–262
Vane JR, Botting RM (1998) Mechanism of action of nonsteroidal anti-inflammatory drugs. Am J Med 104(3S1):2S-8S
Vane J, Botting R (2003) The mechanism of action of aspirin. Thromb Res 110(5–6):255–258
Viegas A, Manso J, Corvo MC, Marques MMB, Cabrita EJ (2011) Binding of ibuprofen, ketorolac, and diclofenac to COX-1 and COX-2 studied by saturation transfer difference NMR. J Med Chem 54(24):8555–8562
Vishvakarma VK, Kumari K, Patel R, Dixit V, Singh P, Mehrotra GK, Chandra R, Chakrawarty AK (2015) Theoretical model to investigate the alkyl chain and anion dependent interactions of gemini surfactant with bovine serum albumin. Spectrochim Acta Part A Mol Biomol Spectrosc 143:319–323
Zarghi A, Ghodsi R (2010) Design, synthesis, and biological evaluation of ketoprofen analogs as potent cyclooxygenase-2 inhibitors. Bioorg Med Chem 18(16):5855–5860. https://doi.org/10.1016/j.bmc.2010.06.094
Zervou M, Andreou A, Goulielmos G, Eliopoulos E (2021) AB0004 the association of the rare RS35667974 IFIH1 gene polymorphism with six autoimmune diseases: structural biological insights. BMJ Publishing Group Ltd
Zhang D, Jia J, Meng L, Xu W, Tang L, Wang J (2010) Synthesis and preliminary antibacterial evaluation of 2-butyl succinate-based hydroxamate derivatives containing isoxazole rings. Arch Pharmacal Res 33(6):831–842
Zidar N, Odar K, Glavac D, Jerse M, Zupanc T, Stajer D (2009) Cyclooxygenase in normal human tissues–is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J Cell Mol Med 13(9b):3753–3763
Acknowledgements
The authors would like to thank An-Najah National University's Faculty of Medicine and Health Sciences for their support.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hawash, M., Jaradat, N., Abualhasan, M. et al. Molecular docking studies and biological evaluation of isoxazole-carboxamide derivatives as COX inhibitors and antimicrobial agents. 3 Biotech 12, 342 (2022). https://doi.org/10.1007/s13205-022-03408-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-022-03408-8