Abstract
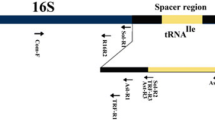
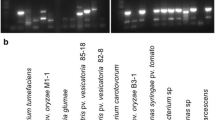
Sensitive and effective phytoplasma DNA amplification in symptomatic rose cultivars is a long unresolved problem. In the present study, improvement in standardization for PCR assay for phytoplasma detection was established with rose samples by selection of various combinations of nested primer pairs of 16S ribosomal gene and secA gene. CTAB DNA extraction method was slightly modified by adding 2% polyvinyl pyrrolidone and increased the isopropanol volume which yielded better quality DNA. Best amplification results were achieved in nested PCR assay employing P1/P7, R16mF2/R16mR2 and R16F2n/R16R2, P1/P7 and R16mF2/R16mR2, and R16mF2/R16mR2 and fU5/rU3 primer pairs. Besides, a multiplex PCR assay was also developed and optimized for consistent identification of phytoplasma in rose samples by employing primer pairs of 16S rRNA and secA genes together in a single PCR reaction by optimizing annealing temperature at 55 °C.




Similar content being viewed by others
References
Abou-Jawdah Y, Karakashian A, Sobh H, Martini M, Lee IM (2002) An epidemic of almond witches’-broom in Lebanon: classification and phylogenetic relationships of the associated phytoplasma. Pl Dis 86(5):477–484
Ahrens U, Seemüller E (1992) Detection of DNA of plant pathogenic mycoplasma like organisms by a polymerase chain reaction that amplifies a sequence of the 16S rRNA gene. Phytopathology 82:828–832
Bellardi MG, Bertaccini A, Madhupriya, Rao GP (2018) Phytoplasma diseases in ornamental crops. In: Rao GP, Bertaccini A, Fiore N, Leiftwing LW (eds) Phytoplasmas: plant pathogenic bacteria-I. Springer, Singapore, pp 191–233
Bertaccini A, Fiore N, Zamorano A, Tiwari AK, Rao GP (2019) Molecular and serological approaches in detection of phytoplasmas in plant and insects. In: Bertaccini A, Oshima A, Kube M, Rao GP (eds) Phytoplasmas: plant pathogenic bacteria-III. Springer, Singapore, pp 105–136
Biswas C, Dey P, Satpathy S (2013) A multiplex nested PCR assay for simultaneous detection of Corchorus golden mosaic virus and a phytoplasma in white jute (Corchorus capsularis L.). Lett Appl Microbiol 56(5):373–378
Clair D, Larrue J, Aubert G, Gillet J, Cloquemin G, Boudon-Padieu E (2003) A multiplex nested-PCR assay for sensitive and simultaneous detection and direct identification of phytoplasma in the Elm yellows group and Stolbur group and its use in survey of grapevine yellows in France. Vitis 42(3):151–157
Deng S, Hiruki C (1991) Amplification of 16S rRNA genes from culturable and nonculturable mollicutes. J Microbiol Methods 14:53–61
Dickinson M, Hodgetts J (2013) PCR analysis of phytoplasma based on the secA gene. In: Dickinson M, Hodgetts J (eds) Phytoplasma: methods and protocols. Springer, New York, pp 205–216
Friar EA (2005) Isolation of DNA from plants with large amount of secondary metabolites. Methods Enzymol 395:3–14
Gholami J, Bahar M, Talebi M (2020) Simultaneous detection and direct identification of phytoplasmas in the aster yellows (16SrI), clover proliferation (16SrVI) and stolbur (16SrXII) groups using a multiplex nested PCR assay in potato plants. Potato Res 63:403–415
Gundersen DE, Lee I-M (1996) Ultrasensitive detection of phytoplasmas by nested-PCR assays using two universal primer pairs. Phytopathol Mediterr 35:144–151
Hodgetts J, Boonham N, Mumford R, Harrison N, Dickinson M (2008) Phytoplasma phylogenetics based on analysis of secA and 23S rRNA gene sequences for improved resolution of candidate species of ‘Candidatus Phytoplasma’. Intl J Syst Evol Microbiol 58(8):1826–1837
Kaminska M, Dziekanowska D, Rudzinâ Ska-langwald A (2001) Detection of phytoplasma infection in rose, with degeneration symptoms. J Phytopathol 149:3–10
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
López MM, Llop P, Olmos A, Marco-Noales E, Cambra M, Bertolini E (2009) Are molecular tools solving the challenges posed by detection of plant pathogenic bacteria and viruses? Curr Issues Mol Biol 11:13–45
Lorenz KH, Schneider B, Ahrens U, Seemüller E (1995) Detection of the apple proliferation and pear decline phytoplasmas by PCR amplification of ribosomal and non ribosomal DNA. Phytopathology 85(7):771–776
Madhupriya, Banyal N, Raju DVS, Manimekalai R, Rao GP, Paul Khurana SM (2017) Association of different groups of phytoplasma in flower malformation, phyllody, foliar yellowing, and little leaf disease of rose (Rosa sp.). J Hort Sci Biotech 92(4):424–431
Malandraki I, Varveri C, Olmos A, Vassilakos N (2015) One-step multiplex quantitative RT-PCR for the simultaneous detection of viroids and phytoplasmas of pome fruit trees. J Virol Methods 213:12–17
Manimekalai R, Soumya VP, Sathish KR, Selvarajan R, Reddy K, Thomas GV, Sasikala M, Rajeev G, Baranwal VK (2010) Molecular detection of 16SrXI group phytoplasma associated with root (wilt) disease of coconut (Cocos nucifera) in India. Pl Dis 94:636
Manimekalai R, Soumya VP, Nair S, Rajeeve GR, Rao GP (2016) PCR detection of phytoplasmas from different tissues of root (wilt) diseased coconut palms. Phytopathogenic Mollicutes 6(1):23–28
NHB Database (2017) Indian horticulture database. NHB, Ministry of Agriculture, Government of India
Omar AF (2016) Association of ‘Candidatus Phytoplasma cynodontis’ with Bermuda grass white leaf disease and its new hosts in Qassim province, Saudi Arabia. J Plant Interact 11(1):101–107
Pallás V, Sánchez-Navarro JA, James D (2018) Recent advances on the multiplex molecular detection of plant viruses and viroids. Front Microbiol 9:2087
Rao GP, Tiwari AK, Kumar S, Baranwal VK (2014) Identification of sugarcane grassy shoots-associated phytoplasma and one of its putative vectors in India. Phytoparasitica 42:349–354
Reddy MG, Rao GP (2019) Development of a multiplex PCR assay for detection and identification of a chickpea phytoplasma strain utilizing 16S rRNA and imp genes specific primers. Phytopathogenic Mollicutes 9(2):257–262
Royal Floraholland (2020) Facts and figures. http://www.statista.com
Swarnalatha P, Reddy MK (2014) Duplex PCR for simultaneous detection of Begomovirus and Phytoplasma from naturally infected tomato. Pest Manag Hortic Ecosyst 20:59–68
Thompson JD, Higgins DG, Gibson TJ (1994) Improved sensitivity of profile searches through the use of sequence weights and gap excision. Comput Appl Biosci 10(1):19–29
Verdin E, Salar P, Danet JL, Choueiri E, Jreijiri F, El Zammar S, Gelie B, Bove JM, Garnier M (2003) ‘Candidatus Phytoplasma phoenicium’ sp. nov., a novel phytoplasma associated with an emerging lethal disease of almond trees in Lebanon and Iran. Int J Sys Evol Microbiol 53(3):833–838
Xu Q, Wen X, Deng X (2004) A simple protocol for isolating genomic DNA from chestnut rose (Rosa roxburghii Tratt) for RFLP and PCR analyses. Plant Mol Biol Rep 22(3):301
Author information
Authors and Affiliations
Contributions
The authors conceived idea, analysed data and drafted manuscript for publication. The first author, TR did all the experiments in the field and lab and also contributed significantly in preparation of draft of the manuscript. N, KPS, MKS and AT contributed equally in preparation and finalization of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there was no conflict of interest.
GenBank submission
All the 16S rRNA and secA gene sequences are submitted to GenBank.
Rights and permissions
About this article
Cite this article
Rihne, T., Namita, Singh, K.P. et al. Improvement in molecular detection of phytoplasma associated with rose by selection of suitable primers and development of a multiplex PCR assay. 3 Biotech 11, 190 (2021). https://doi.org/10.1007/s13205-021-02713-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02713-y