Abstract
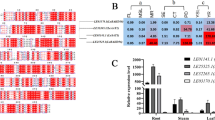
Gamma-tocopherol methyltransferase (γ-TMT) converts γ-toc to α-toc—the rate limiting step in toc biosynthesis. Sequencing results revealed that the coding regions of γ-TMT1 and γ-TMT3 were strongly similar to each other (93% at amino acid level). Based on the differences in the N-terminal amino acids, Glycine max-γ-TMT proteins are categorized into three isoforms: γ-TMT1, 2 and 3. In silico structural analysis revealed the presence of chloroplast transit peptide (cTP) in γ-TMT1 and γ-TMT3 protein. However, other properties of transit peptide like presence of hydrophobic amino acids at the first three positions of N-terminal end and lower level of acidic amino acids were revealed only in γ-TMT3 protein. Subcellular localization of GFP fused γ-TMT1 and γ-TMT3 under 35S promoter was studied in Nicotiana benthamiana using confocal microscopy. Results showed that γ-TMT1 was found in the cytosol and γ-TMT3 was found to be localized both in cytosol and chloroplast. Further the presence γ-TMT3 in chloroplast was validated by quantifying α-tocopherol through UPLC. Thus the present study of cytosolic localization of the both γ-TMT1 and γ-TMT3 proteins and chloroplastic localization of γ-TMT3 will help to reveal the importance of γ-TMT encoded α-toc in protecting both chloroplastic and cell membrane from plant oxidative stress.





Similar content being viewed by others
References
Arun M, Subramanyam K, Theboral J, Sivanandhan G, Rajesh M, Dev GK, Ganapathi A (2014) Transfer and targeted overexpression of γ-tocopherol methyltransferase (γ-TMT) gene using seed-specific promoter improves tocopherol composition in Indian soybean cultivars. Appl Biochem Biotechnol 172(4):1763–1776
Azzi A, Stocker A (2000) Vitamin E: non-antioxidant roles. Prog Lipid Res 39(3):231–255
Bergmüller E, Porfirova S, Dörmann P (2003) Characterization of an Arabidopsis mutant deficient in γ-tocopherol methyltransferase. Plant Mol Biol 52(6):1181–1190
Botha FC, Black KG (2000) Sucrose phosphate synthase and sucrose synthase activity during maturation of internodal tissue in sugarcane. Funct Plant Biol 27(1):81–85
Bruce BD (2000) Chloroplast transit peptides: structure, function and evolution. Trends Cell Biol 10(14):40–447
Chen DF, Zhang M, Wang YQ, Chen XW (2012) Expression of γ-tocopherol methyltransferase gene from Brassica napus increased α-tocopherol content in soybean seed. Biol Plant 56(1):131–134
Cho EA, Lee CA, Kim YS, Baek SH, Reyes BG, Yun SJ (2005) Expression of γ-tocopherol methyltransferase transgene improves tocopherol composition in lettuce (Latuca sativa L.). Mol Cells 19(1):16–22
DellaPenna D, Pogson BJ (2006) Vitamin synthesis in plants: tocopherols and carotenoids. Annu Rev Plant Biol 57:711–738
DellaPenna D (2005) A decade of progress in understanding vitamin E synthesis in plants. J Plant Physiol 162:729–737
Drevon CA (1991) Absorption, transport and metabolism of vitamin E. Free Radic Res Commun 14(4):229–246
Dwiyanti MS, Yamada T, Sato M, Abe J, Kitamura K (2011) Genetic variation of γ-tocopherol methyltransferase gene contributes to elevated α-tocopherol content in soybean seeds. BMC Plant Biol 11(1):152
Frigerio L, de Virgilio M, Prada A, Faoro F, Vitale A (1998) Sorting of phaseolin to the vacuole is saturable and requires a short C-terminal peptide. Plant Cell 10(6):1031–1042
Fryer MJ (1992) The antioxidant effects of thylakoid vitamin E (α-tocopherol). Plant Cell Environ 15(4):381–392
Ghimire BK, Seong ES, Lee CO, Lim JD, Lee JG, Yoo JH, Chung IM, Kim NY, Yu CY (2011) Enhancement of alpha-tocopherol content in transgenic Perilla frutescens containing the gamma-TMT gene. Afr J Biotechnol 10:2430–2439
Guo J (2006) Overexpression of VTE1 from Arabidopsis resulting in high vitamin E accumulation and salt stress tolerance increase in tobacco plant. Chin J Appl Environ Biol 12(4):468
Hass CG, Tang S, Leonard S, Traber MG, Miller JF, Knapp SJ (2006) Three non-allelic epistatically interacting methyltransferase mutations produce novel tocopherol (vitamin E) profiles in sunflower. Theor Appl Genet 113(5):767–782
Horvath G, Wessjohann L, Bigirimana J, Jansen M, Guisez Y, Caubergs R, Horemans N (2006) Differential distribution of tocopherols and tocotrienols in photosynthetic and non-photosynthetic tissues. Phytochemistry 67(12):1185–1195
Hosomi A, Arita M, Sato Y, Kiyose C, Ueda T, Igarashi O, Inoue K (1997) Affinity for α-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett 409(1):105–108
Hofius D, Hajirezaei MR, Geiger M, Tschiersch H, Melzer M, Sonnewald U (2004) RNAi-mediated tocopherol deficiency impairs photoassimilate export in transgenic potato plants. Plant Physiol 135:1256–1268
Jin S, Daniell H (2014) Expression of γ-tocopherol methyltransferase in chloroplasts results in massive proliferation of the inner envelope membrane and decreases susceptibility to salt and metal-induced oxidative stresses by reducing reactive oxygen species. Plant Biotechnol J 12(9):1274–1285
Lee DW, Lee S, Lee GJ, Lee KH, Kim S, Cheong GW, Hwang I (2006) Functional characterization of sequence motifs in the transit peptide of Arabidopsis small subunit of rubisco. Plant Physiol 140(2):466–483
Lehtimäki N, Koskela MM, Dahlström KM, Pakula E, LintalaM SM, Hippler M, Hanke GT, RokkaA BN, Salminen TA (2014) Posttranslational modifications of FERREDOXIN-NADP+ OXIDOREDUCTASE in Arabidopsis chloroplasts. Plant Physiol 166(4):1764–1776
Leister D (2003) Chloroplast research in the genomic age. Trends Genet 19(1):47–56
Lichtenthaler HK, Buschmann C, Döll M, Fietz HJ, Bach T, Kozel U, Rahmsdorf U (1981) Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth Res 2(2):115–141
López-Juez E (2006) Plastid biogenesis, between light and shadows. J Exp Bot 58(1):11–26
Mène-Saffrané L, Jones AD, DellaPenna D (2010) Plastochromanol-8 and tocopherols are essential lipid-soluble antioxidants during seed desiccation and quiescence in Arabidopsis. Proc Natl Acad Sci 107(41):17815–17820
Millar AH, Whelan J, Small I (2006) Recent surprises in protein targeting to mitochondria and plastids. Curr Opin Plant Biol 9(6):610–615
Miras S, Salvi D, Piette L, Seigneurin BD, Grunwald D, Reinbothe C, Joyard J, Reinbothe S, Rolland N (2007) Toc159-and Toc75-independent import of a transit sequence-less precursor into the inner envelope of chloroplasts. J Biol Chem 282(40):29482–29492
Munné-Bosch S (2005) The role of α-tocopherol in plant stress tolerance. J Plant Physiol 162(7):743–748
Munné-Bosch S, Alegre L (2002a) The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci 21(1):31–57
Munné-Bosch S, Alegre L (2002b) Plant aging increases oxidative stress in chloroplasts. Planta 214(4):608–615
Munné-Bosch S, Schwarz K, Alegre L (1999) Enhanced formation of α-tocopherol and highly oxidized abietane diterpenes in water-stressed rosemary plants. Plant Physiol 121(3):1047–1052
Munné-Bosch S, Weiler EW, Alegre L, Müller M, Düchting P, Falk J (2007) α-Tocopherol may influence cellular signaling by modulating jasmonic acid levels in plants. Planta 225(3):681–691
Patron NJ, Waller RF (2007) Transit peptide diversity and divergence: a global analysis of plastid targeting signals. BioEssays 29(10):1048–1058
Richly E, Leister D (2004) An improved prediction of chloroplast proteins reveals diversities and commonalities in the chloroplast proteomes of Arabidopsis and rice. Gene 329:11–16
Russin WA, Evert RF, Vanderveer PJ, Sharkey TD, Briggs SP (1996) Modification of a specific class of plasmodesmata and loss of sucrose export ability in the sucrose export defective1 maize mutant. Plant Cell 8(4):645–658
Sakamoto W, Miyagishima SY, Jarvis P (2008) Chloroplast biogenesis: control of plastid development, protein import, division and inheritance. Arabidopsis book 6, e0110
Sattler SE, Gilliland LU, Magallanes LM, Pollard M, DellaPenna D (2004) Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 16(6):1419–1432
Schneider C (2005) Chemistry and biology of vitamin E. Mol Nutr Food Res 49(1):7–30
Shintani D, DellaPenna D (1998) Elevating the vitamin E content of plants through metabolic engineering. Science 282(5396):2098–2100
Sies H, Menck CF (1992) Singlet oxygen induced DNA damage. Mutat Res DNAging 275(3–6):367–375
Skovsen E, Snyder JW, Lambert JD, Ogilby PR (2005) Lifetime and diffusion of singlet oxygen in a cell. J Phys Chem B 109(18):8570–8573
Soll J, Schultz G, Joyard J, Douce R, Block MA (1985) Localization and synthesis of prenylquinones in isolated outer and inner envelope membranes from spinach chloroplasts. Arch Biochem Biophys 238(1):290–299
Stocker A, Azzi A (2000) Tocopherol-binding proteins: their function and physiological significance. Antioxid Redox Signal 2(3):397–404
Sun S, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li G (2004) Monodisperse mfe2o4 (m = fe, co, mn) nanoparticles. J Am Chem Soc 126(1):273–279
Szymańska R, Kruk J (2008a) γ-Tocopherol dominates in young leaves of runner bean (Phaseolus coccineus) under a variety of growing conditions: the possible functions of γ-tocopherol. Phytochemistry 69:2142–2148
Szymańska R, Kruk J (2008b) Tocopherol content and isomers' composition in selected plant species. Plant Physiol Biochem 46(1):29–33
Tavva VS, Kim YH, Kagan IA, Dinkins RD, Kim KH, Collins GB (2007) Increased α-tocopherol content in soybean seed overexpressing the Perilla frutescens γ-tocopherol methyltransferase gene. Plant Cell Rep 26(1):61–70
Taylor SL, Lamden MP, Tappel AL (1976) Sensitive fluorometric method for tissue tocopherol analysis. Lipids 11(7):530–538
Traber MG, Sies H (1996) Vitamin E in humans: demand and delivery. Annu Rev Nutr 16(1):321–347
Van Wijk KJ (2004) Plastid proteomics. Plant Physiol Biochem 42(12):963–977
Villarejo A, Burén S, Larsson S, Déjardin A, Monné M, Rudhe C, Von Heijne G (2005) Evidence for a protein transported through the secretory pathway en route to the higher plant chloroplast. Nat Cell Biol 7(12):1224
Vinutha T, Bansal N, Prashat GR, Krishnan V, Kumari S, Dahuja A, Sachdev A, Rai RD (2015) Three dimensional structure prediction and in silico functional analysis of gamma tocopherol methyl transferase from Glycine max. Int J Bioinform Res 6:289–299
Vinutha T, Bansal N, Kumari K, Rama Prashat G, Sreevathsa R, Krishnan V, Kumari S, Dahuja A, Lal S, Sachdev A, Praveen S (2017) Comparative analysis of tocopherol biosynthesis genes and its transcriptional regulation in soybean seeds. J Agric Food Chem 65(50):11054–11064
Von Heijne G, Steppuhn J, Hermann SG (1989) Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem 80:535–545
Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low temperature, or high-salt stress. Plant Cell 6:251–264
Yusuf MA, Sarin NB (2007) Antioxidant value addition in human diets: genetic transformation of Brassica juncea with γ-TMT gene for increased α-tocopherol content. Transgenic Res 16(1):109–113
Zybailov B, Rutschow H, Friso G, Rudella A, Emanuelsson O, Sun Q, van Wijk KJ (2008) Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS One 3(4):1994
Acknowledgements
The authors are thankful for financial support mainly from DST-SERB, Receipt Number-No. SB/389 YS/LS-100/2013 and also thankful for great support from ICAR- IARI Fund.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All the authors whose names are listed just belowthe manuscript title clearly certify that they have NO involvement in or affiliations with organization or entity with any type of financial or non-financial interest in the subject matter or materials described or discussed in this current manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumari, K., Rai, M.P., Bansal, N. et al. Study of subcellular localization of Glycine max γ-tocopherol methyl transferase isoforms in N. benthamiana. 3 Biotech 10, 110 (2020). https://doi.org/10.1007/s13205-020-2086-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-2086-9