Abstract
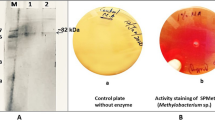
The Trichoderma harzianum l-methioninase was purified 7.15-fold with a recovery of 47.9% and the specific activity of 74.4 U/mg of protein. The purified enzyme has an apparent molecular mass of 48 kDa on SDS-PAGE and exhibited maximum activity at pH 8 and 35 °C. The enzyme was catalytically stable below 50 °C and at a pH range of 6.0–8.5. The thermal inactivation of l-methioninase exhibited first-order kinetics with the k value between 5.71 × 10–4 min−1 and 1.83 × 10–2 min−1. The studies on thermodynamic parameters of l-methioninase indicated the compaction and aggregation of the enzyme molecule during denaturation. This is the first report of thermodynamic analysis of thermal inactivation in l-methioninase. The enzyme activity was enhanced by Li+ and inhibited by Cu2+, Co2+, Fe2+, Hydroxylamine and PMSF. The purified enzyme showed Km, Vmax and kcat value of 1.19 mM, 21.27 U/mg/min and 16.11 s−1, respectively. The l-methioninase inhibited the growth of human cell lines hepatocellular carcinoma (Hep-G2) and breast carcinoma (MCF-7) with IC50 values of 14.12 μg/ml and 20.07 μg/ml, respectively. The in vivo antitumor activity of l-methioninase was evaluated against DAL cell lines bearing in Swiss albino mice. The enzyme effectively reduced tumor volume, packed cell volume, viable cell count and restored hematological parameters, serum enzyme and lipid profile to normal levels compared to DAL control mice. The present study has demonstrated the high efficacy of Trichoderma harzianum l-methioninase against cancer cell lines in vitro and in vivo conditions. The purified l-methioninase has significant thermal stability and better catalytic properties than the enzyme purified from other sources.





Similar content being viewed by others
References
Abu-Tahon MA, Isaac GS (2016) Purification and characterization of new alkaline L-methioninase from Aspergillus ustus AUMC 1051 grown under solid-state fermentation conditions. Egypt J Bot 56:785–798
Bertoldi M, Cellini B, Clausen T, Voltattorni CB (2002) Spectroscopic and kinetic analyses reveal the pyridoxal 5 ‘-phosphate binding mode and the catalytic features of Treponema denticola cystalysin. Biochemistry 41:9153–9164
Bhatti HN, Madeeha M, Asgher M, Batool N (2006) Purification and thermodynamic characterization of glucose oxidase from a newly isolated strain of Aspergillus niger. Can J Microbiol 52:519–524
Cellarier E, Durando X, Vasson MP, Farges MC, Demiden A, Maurizis JC, Madelmont JC, Chollet P (2003) Methionine dependency and cancer treatment. Cancer Treat Rev 29:489–499
Dias B, Weimer B (1998) Purification and characterization of L-methionine γ-lyase from Brevibacterium linens BL2. AppI Environ Microbiol 64:3327–3331
El Awady ME, Selim MS, Abd El-Razek AS, Asker M (2017) Production, purification and characterization of L-methioninase from Streptomyces variabilis 3MA2016. Res J Pharmaceut Biol Chem Sci 8:906–921
El-Sayed ASA (2010) Microbial L-methioninase, molecular characterization, and therapeutic applications. Appl Microbiol Biotechnol 86:445–467
El-Sayed ASA (2011) Purification and characterization of new l-methioninase from solid cultures of Aspergillus flavipes. J Microbiol 49:130–140
El-Sayed AS, Shouman SA, Nassrat HM (2012) Pharmacokinetics, immunogenicity and anticancer efficiency of Aspergillus flavipes L-methioninase. Enzyme Microb Technol 51:200–210
El-Sayed AS, Ruff LE, Ghany SE, Ali GS, Esener S (2017) Molecular and spectroscopic characterization of Aspergillus flavipes and Pseudomonas putida L-Methionine γ-Lyase in vitro. Appl Biochem Biotechnol 181:1513–1532
Esaki N, Soda K (1987) L-Methionine γ-lyase from Pseudomonas putida and Aeromonas. Method Enzymol 143:459–465
Fenninger LD, Mider GB (1954) Energy and nitrogen metabolism in cancer. Adv Cancer Res 2:229–253
Gummadi SN (2003) What is the role of thermodynamics on protein stability? Biotechnol Bioprocess E 8:9–18
Guo H, Lishko VK, Herrera H, Groce A, Kubota T, Hoffman RM (1993) Therapeutic tumor-specific cell cycle block induced by methionine starvation in vivo. Cancer Res 53:5676–5679
Halpern BC, Clark BR, Hardy DN, Halpern RM, Smith RA (1974) The effect of replacement of methionine by homocystine on survival of malignant and normal adult mammalian cells in culture. Proc Natl Acad Sci 71:1133–1136
Hogland HC (1982) Haematological complication of cancer chemotherapy. Semin Oncol 9:95–102
Ito S, Nakamura T, Eguchi Y (1976) Purification and characterization of methioninase from Pseudomonas putida. J Biochem 79:1263–1272
Kamran A, Rehman HU, Qader SAU, Baloch AH, Kamal M (2015) Purification and characterization of thiol dependent, oxidation-stable serine alkaline protease from thermophilic Bacillus sp. J Genet Eng Biotechnol 13:59–64
Kapat A, Panda T (1996) pH and thermal stability studies of chitinase from Trichoderma harzianum. Bioprocess Eng 16:269–272
Kawaguchi K, Igarashi K, Li S, Han Q, Tan Y, Miyake K, Kiyuna T, Miyake M, Murakami T, Chmielowski B, Nelson SD (2018) Recombinant methioninase (rMETase) is an effective therapeutic for BRAF-V600E-negative as well as-positive melanoma in patient-derived orthotopic xenograft (PDOX) mouse models. Oncotarget 9:915
Kreis W, Hession C (1973) Isolation and purification of L-methionine- α-deamino-γ mercapto-methane-lyase (L-methioninase) from Clostridium sporogenes. Cancer Res 33:1862–1865
Laakso S, Nurmikko V (1976) A spectroscopic assay for demethiolating activity. Anal Biochem 72:600–605
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage. Nature 227:680–685
Lockwood BC, Coombs GH (1991) Purification and characterization of methionine γ-lyase from Trichomonas vaginalis. Biochem J 279:675–682
Lowry OH, Rosebrough NJ, Farr AL, Randall RL (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–273
Manukhov I, Mamaeva DV, Rastorguev SM, Faleev NG, Morozova EA, Demidkina TV, Zavilgelsky GB (2005) A gene encoding Lmethionine γ-lyase is present in Enterobacteriaceae family genomes: identification and characterization of Citrobacter freundii L-methionine γ-lyase. J Bacteriol 187:3889–3893
Marin E, Sanchez L, Perez MD, Puyol P, Calvo M (2003) Effect of heat treatment on bovine lactoperoxidase activity in skim milk: kinetic and thermodynamic analysis. J Food Sci 68:89–93
Nakayama T, Esaki N, Lee WJ, Tanaka I, Tanaka H, Soda K (1984) Purification and properties of L-methioninase γ-lyase from Aeromonas sp. Agric Biol Chem 48:2367–2369
Narwal RK, Bhushan B, Pal A, Panwar A, Malhotra S (2016) Purification, physico-chemico-kinetic characterization and thermal inactivation thermodynamics of milk clotting enzyme from Bacillus subtilis MTCC 10422. LWT-Food Sci Technol 65:652–660
Nikulin A, Revtovich S, Morozova E, Nevskaya N, Nikonov S, Garber M, Demidkina T (2008) High-resolution structure of methionine γ-lyase from Citrobacter freundii. Acta Crystallogr D 64:211–218
Pal A, Khanum F (2011) Characterizing and improving the thermostability of purified xylanase from Aspergillus niger DFR-5 grown on solid-state-medium. J Biochem Technol 2:203–209
Pavani K, Saradhi SV (2014) Cloning and expression of methionine-γ-lyase (MGL) of Brevibacterium linens. Int J Curr Microbiol Appl Sci 3:615–631
Prasad SB, Giri A (1994) Antitumor effect of cisplatin against murine ascites Dalton’s Lymphoma. Indian J Exp Biol 32:155–162
Price VE, Greenfield RE (1958) Anemia in cancer. In: Greenstein JP, Haddow A (eds) Advances in cancer research. Academic Press, New York, pp 199–200
Ronda L, Bazhulina NP, Morozova EA, Revtovich SV, Chekhov VO, Nikulin AD, Demidkina TV, Mozzarelli A (2011) Exploring methionine γ-lyase structure-function relationship via microspectrophotometry and X-ray crystallography. Biochim Biophys Acta Proteins Proteom 1814:834–842
Ruiz-Herrera J, Starkey RL (1969) Dissimilation of methionine by fungi. J Bacteriol 99:544–551
Salim N, Santhiagu A, Joji K (2019) Process modelling and optimization of high yielding L-Methioninase from a newly isolated Trichoderma harzianum using Response Surface methodology and artificial neural network coupled genetic algorithm. Biocatal Agric Biotechnol 17:299–308
Selim MH, Karm Eldin EZ, Saad MM, Mostafa ESE, Shetia YH, Anise AAH (2015) Purification, characterization of L-methioninase from Candida tropicalis, and its application as an anticancer. Biotechnol Res Int. https://doi.org/10.1155/2015/173140
Selim MH, Elshikh HH, Saad MM, Mostafa EE, Mahmoud MA (2016) Purification and characterization of a novel thermo stable L-methioninase from Streptomyces sp. DMMMH4 and its evaluation for anticancer activity. J Appl Pharm Sci 6:53–60
Sun X, Yang Z, Li S, Tan Y, Zhang N, Wang X, Yagi S, Yoshioka T, Takimoto A, Mitsushima K, Suginaka A, Frankel EP, Hoffman RM (2003) In vivo efficiency of recombinant methioninase is enhanced by the combination of polyethylene glycol conjugation and pyridoxal 5-phosphate supplementation. Cancer Res 63:8377–8383
Sundar WA, Nellaiah H (2013) Production of methioninase from Serratia marcescens isolated from soil and its anti-cancer activity against Dalton’s Lymphoma Ascitic (DLA) and Ehrlich Ascitic Carcinoma (EAC) in swiss albino mice. Trop J Pharm Res 12:699–704
Tan Y, Sun X, Xu M, An Z, Tan X, Tan X, Han Q, Miljkovic DA, Yang M, Hoffman RM (1998) Polyethylene glycol conjugation of recombinant methioninase for cancer therapy. Protein Expres and Purif 12:45–52
Tan Y, Xu M, Hoffman RM (2010) Broad selective efficacy of recombinant methioninase and polyethylene glycol-modified recombinant methioninase on cancer cells in vitro. Anticancer Res 30:1041–1046
Tanaka H, Esaki N, Soda K (1977) Properties of L-methionine γ-lyase from Pseudomonas ovalis. Biochemistry 16:100–106
Tanaka H, Esaki N, Soda K (1985) A versatile bacterial enzyme: methionine γ-lyase. Enzyme Microb Technol 7:530–536
Wise DR, Thompson CB (2010) Glutamine addiction: a new therapeutic target in cancer. Trends biochem sci 35:427–433
Acknowledgements
One of the authors Ms. Nisha Salim is thankful to UGC for providing research fellowship. The authors wish to acknowledge Dr K. Rathinasamy, and Ms Shabeeba of National institute of Technology Calicut for providing facilities for cell culture studies.
Author information
Authors and Affiliations
Contributions
AS conceived the original idea and designed the study. NS performed the experiment and wrote the paper. KJ provided help in performing the experiment.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest. All the authors consent to the submission of this manuscript to the journal.
Ethical approval
All the procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted and ethical approval was obtained from Committee for the purpose of control and supervision of experiments on animals (CPCSEA). (Reg No: 688/PO/Re/S/02/CPCSEA.), Approval No: NCP/IAE/2018-19/29.
Additional information
Accession number
The rDNA sequence of Trichoderma harzianum was deposited to gene bank under accession number MH828332.1.
Rights and permissions
About this article
Cite this article
Salim, N., Santhiagu, A. & Joji, K. Purification, characterization and anticancer evaluation of l-methioninase from Trichoderma harzianum. 3 Biotech 10, 501 (2020). https://doi.org/10.1007/s13205-020-02494-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02494-w