Abstract
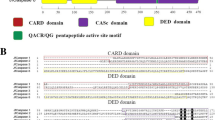
Among various caspases, caspase-9 plays a crucial role in the initiation phase of apoptotic cascade. To investigate about it in a high-valued freshwater fish species rohu (Labeo rohita), we cloned and characterized full-length caspase-9 cDNA (Lrcasp9) and analyzed its expression following bacterial infections and anti-viral vaccinations. The Lrcasp9 consisted of 1619-bp nucleotides (nt) having an ORF of 1302 nt encoding a polypeptide of 433 amino acids (aa) with a molecular mass of ∼ 48.20 kDa. Structurally, Lrcasp9 comprised of one CARD domain (1–89 aa) and one CASc domain (161–430 aa). The CASc domain consisted of one large subunit (p20) spanning from 168 to 300 aa, and a small sub unit (p10) from 343 to 430 aa. The caspase family signature histidine active motif H233SAYDCCVVIILSHG247, cysteine active motif K287PKLFFIQACGG298 and pentapeptide “QACGG” active sites present in the p20 domain of Lrcasp9 was conserved across fish species, mouse and human caspase-9. Phylogenetically, it was closely related to common carp caspase-9 and exhibited significant similarity (90.1%) and identity (85.3%) in their amino acid sequence. In the uninfected fish, Lrcasp9 gene expression was highest (~ 5.3-fold) in blood and lowest in gill. In response to Aeromonas hydrophila and Edwardsiella tarda infection and rhabdoviral vaccination, Lrcasp9 gene expression was significantly (p > 0.05) enhanced in gill, liver, kidney and spleen, and also in vitro during cell death, suggesting activation of the intrinsic apoptotic pathway in bacterial infections and anti-viral vaccination in Labeo rohita.








Similar content being viewed by others
References
Ahne W, Bjorklund HV, Essbauer S et al (2002) Spring viremia of carp (SVC). Dis Aquat Organ 52(3):261–272
Al-Hussinee L, Lord S, Stevenson RM et al (2011) Immunohistochemistry and pathology of multiple Great Lakes fish from mortality events associated with viral hemorrhagic septicemia virus type IVb. Dis Aquat Organ 93(2):117–127
Altschul SF, Gish W, Miller W et al (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Angelastro JM, Moon NY, Liu DX et al (2001) Characterization of a novel isoform of caspase-9 that inhibits apoptosis. J Biol Chem 276(15):12190–12200
Banerjee R, Patel B, Basu M et al (2017) Molecular cloning, characterization and expression of immunoglobulin D (IgD) on pathogen challenge and PAMPs stimulation in freshwater carp, Catla catla. Microbiol Immunol 61(10):452–458
Basu M, Lenka SS, Paichha M et al (2016) Immunoglobulin (Ig) D in Labeo rohita is widely expressed and differentially modulated in viral, bacterial and parasitic antigenic challenges. Vet Immunol Immunopathol 179:77–84
Bitzer M, Armeanu S, Prinz F et al (2002) Caspase-8 and Apaf-1-independent caspase-9 activation in Sendai virus-infected cells. J Biol Chem 277(33):29817–29824
Boatright KM, Salvesen GS (2003) Mechanisms of caspase activation. Curr Opin Cell Biol 15(6):725–731
Bootland LM, Leong JC (1999) Infectious haematopoietic necrosis virus. In: Woo PTK, Bruno DW (eds) Fish diseases and disorders, vol 3. CAB International, Wallingford, pp 57–121
Duan H, Orth K, Chinnaiyan AM et al (1996) ICE-LAP6, a novel member of the ICE/Ced-3 gene family, is activated by the cytotoxic T cell protease granzyme B. J Biol Chem 271(28):16720–16724
Earnshaw WC, Martins LM, Kaufmann SH (1999) Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 68:383–424
Gao D, Xu Z, Zhang X et al (2013) Molecular cloning, immunohistochemical localization, characterization and expression analysis of caspase-9 from the purse red common carp (Cyprinus carpio) exposed to cadmium. Aquat Toxicol 142–143:53–62
Hakem R, Hakem A, Duncan GS et al (1998) Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell 94(3):339–352
Hofmann K, Bucher P, Tschopp J (1997) The CARD domain: a new apoptotic signalling motif. Trends Biochem Sci 22(5):155–156
Karunasagar I, Rosalind G, Gopal M et al (1989) Aeromonas hydrophila septicemia of Indian major carps in some commercial fish farms of West Godavari district, Andhra Pradesh. Curr Sci 58:1044–1045
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis (a basic biological phenomenon with wide-ranging implications in tissue kinetics). Br J Cancer 26(4):239–257
Krumschnabel G, Podrabsky JE (2009) Fish as model systems for the study of vertebrate apoptosis. Apoptosis 14(1):1–21
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) method. Methods 25:402–408
Majeed AS, Nambi KS, Taju G et al (2013) Establishment and characterization of permanent cell line from gill tissue of Labeo rohita (Hamilton) and its application in gene expression and toxicology. Cell Biol Toxicol 29:59–73
Mork C, Hershberger P, Kocan R et al (2004) Isolation and characterization of a rhabdovirus from starry flounder (Platichthys stellatus) collected from the northern portion of Puget Sound, Washington, USA. J Gen Virol 85:495–505
Mu Y, Xiao X, Zhang J et al (2010) Molecular cloning and functional characterization of caspase-9 in large yellow croaker (Pseudosciaena crocea). Dev Comp Immunol 34(3):300–307
Reis MI, do Vale A, Pinto C et al (2007) First molecular cloning and characterisation of caspase-9 gene in fish and its involvement in a gram negative septicaemia. Mol Immunol 44(7):1754–1764
Renatus M, Stennicke HR, Scott FL et al (2001) Dimer formation drives the activation of the cell death protease caspase-9. Proc Natl Acad Sci USA 98(25):14250–14255
Sakamaki K, Nozaki M, Kominami K et al (2007) The evolutionary conservation of the core components necessary for the extrinsic apoptotic signaling pathway in Medaka fish. BMC Genom 8:141
Saleh A, Srinivasula SM, Acharya S et al (1999) Cytochrome c and dATP-mediated oligomerization of Apaf-1 is a prerequisite for procaspase-9 activation. J Biol Chem 274(25):17941–17945
Salvesen GS, Dixit VM (1997) Caspases: intracellular signalling by proteolysis. Cell 91(4):443–446
Shome BR, Shome R, Sarangi N et al (1996) Etiological characterization of acute infectious abdominal dropsy outbreak affecting Indian major carp, Cirrhinus mrigala in South Andaman. Curr Sci 70:744–747
Srinivasula SM, Fernandes-Alnemri T, Zangrilli J et al (1996) The Ced-3/interleukin 1beta converting enzyme-like homolog Mch6 and the lamin-cleaving enzyme Mch2alpha are substrates for the apoptotic mediator CPP32. J Biol Chem 271(43):27099–27106
Srinivasula SM, Hegde R, Saleh A et al (2001) A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 410(6824):112–116
Strasser A, O’Connor L, Dixit VM (2000) Apoptosis signalling. Annu Rev Biochem 69:217–245
Tamura K, Dudley J, Nei M et al (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Whitfiel AE, Calisher CH, Stone DM et al (2011) Rhabdoviridae. In: King MQ, Adams MJ, Carstens EB, Lefkowitz EJ (eds) Virus taxonomy, ninth report of the International Committee on taxonomy of viruses. Elsevier, Oxford, pp 686–714
Acknowledgements
This work was supported by the grant of National Agricultural Science Fund (NASF) of the Indian Council of Agricultural Research (ICAR) (project code: NASF/BS-4003). The authors express their sincere thanks and gratitude to the Director, ICAR-CIFA and HOD, FHMD for providing necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Giri, A.K., Paichha, M., Saha, A. et al. Lrcasp9 shares similarity in structural motifs with human caspase-9 and is activated following bacterial infection and anti-viral vaccination. 3 Biotech 8, 340 (2018). https://doi.org/10.1007/s13205-018-1366-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1366-0