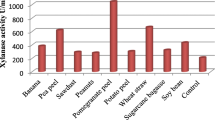
Abstract
Improved xylanase production was carried out through optimization of environmental stresses during spore preservation, seed cultivation and batch fermentation and identifies the markers at various stages. The maximum spore size (radius 6.5 µm) of Aspergillus niger was noticed after 28 days of spore preservation. During seed cultivation, the hypha formed alongside of germination tube (length 196.8 µm) was noticed only at pH-7 after 18 h of incubation at 28 °C. Therefore, pH-7 and 28 °C were considered as optimum during seed cultivation. In this stage, the final pH of the medium was found to be 6.2 which can be used as marker for completion of seed culture. The production media was optimized through Taguchi methodology. The maximum xylanase production was found to be 1575.93 U. The optimum concentration for media components was found to be xylan from beechwood of 3 g/l, potassium nitrate of 10 g/l, magnesium sulphate of 5 g/l, di-potassium hydrogen phosphate of 50 mM, calcium carbonate of 2 g/l, 1000× of trace element (1 ml) and sodium chloride of 5 g/l. It is evident that improved production of xylanase can be possible through optimization of environmental stresses during spore preservation, seed cultivation and batch fermentation and can be intensified through identification of markers at various stages of fermentation process.



Similar content being viewed by others
References
Ahmed S, Riaz S, Jamil A (2009) Molecular cloning of fungal xylanases: an overview. Appl Microbiol Biotechnol 84(1):19–35. https://doi.org/10.1007/s00253-009-2079-4
Anderson J, Smith J (1972) The effects of elevated temperatures on spore swelling and germination in Aspergillus niger. Can J Microbiol 18(3):289–297
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23(3):257–270
Basak B, Bhunia B, Dutta S, Dey A (2013) Enhanced biodegradation of 4-chlorophenol by Candida tropicalis PHB5 via optimization of physicochemical parameters using Taguchi orthogonal array approach. Int Biodeter Biodegrad 78:17–23
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Colin VL, Baigorí MD, Pera LM (2013) Tailoring fungal morphology of Aspergillus niger MYA 135 by altering the hyphal morphology and the conidia adhesion capacity: biotechnological applications. AMB Express 3(27):1–13
Dague E, Alsteens D, Latgé J-P, Dufrêne YF (2008) High-resolution cell surface dynamics of germinating Aspergillus fumigatus conidia. Biophys J 94(2):656–660
Dantigny P, Nanguy SP-M (2009) Significance of the physiological state of fungal spores. Int J Food Microbiol 134(1):16–20
Knob A, Terrasan C (2010) Carmona E. β-Xylosidases from filamentous fungi: an overview. World J Microbiol Biotechnol 26:389–407
Kumar A, Bhunia B, Dasgupta D, Mandal T, Dey A, Datta S, Bhattacharya P (2013) Optimization of culture condition for growth and phenol degradation by Alcaligenes faecalis JF339228 using Taguchi Methodology. Desalin Water Treat 51(16–18):3153–3163
Marleau J, Dalpé Y, St-Arnaud M, Hijri M (2011) Spore development and nuclear inheritance in arbuscular mycorrhizal fungi. BMC Evol Biol 11(1):51
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Mitra A (1998) Fundamentals of quality control and improvement. Pearson Educational Asia, Delhi
Osherov N, May GS (2001) The molecular mechanisms of conidial germination. FEMS Microbiol Lett 199(2):153–160
Sae-lee N (2011) The production of fungal mannanase, cellulase and xylanase using palm kernel meal as a substrate. Walailak J Sci Technol 4(1):67–82
Sakthiselvan P, Naveena B, Partha N (2014) Molecular characterization of a Xylanase-producing fungus isolated from fouled soil. Braz J Microbiol 45(4):1293–1302
Taguchi G (1986) Introduction to quality engineering. Asian Productivity Organization. American Supplier Institute Inc., Dearborn
Uday USP, Bandyopadhyay TK, Bhunia B (2016a) Optimization of nutritional and physiochemical requirements on acidic xylanase production from Aspergillus niger KP874102.1. Mater Today Proc 3(10):3367–3374
Uday USP, Bandyopadhyay TK, Bhunia B (2016b) Rapid development of xylanase assay conditions using Taguchi methodology. Bioengineered 7(6):424–431
Uday USP, Choudhury P, Bandyopadhyay TK, Bhunia B (2016c) Classification, mode of action and production strategy of xylanase and its application for biofuel production from water hyacinth. Int J Biol Macromol 82:1041–1054
Uday USP, Majumdar R, Tiwari ON, Mishra U, Mondal A, Bandyopadhyay TK, Bhunia B (2017) Isolation, screening and characterization of a novel extracellular xylanase from Aspergillus niger (KP874102. 1) and its application in orange peel hydrolysis. Int J Biol Macromol 105:401–409
Zhou J, Wu D, Guo D (2010) Optimization of the production of thiocarbohydrazide using the Taguchi method. J Chem Technol Biotechnol 85:1402–1406
Funding
This material is based upon work supported by the National Institute of Technology, Agartala, India. The authors would like to acknowledge the National Institute of Technology, Agartala, Ministry of Human Resource and Development, Government of India, for Fellowship (0000-0003-4637-991X).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Uday, U.S.P., Goswami, S., Gopikrishna, K. et al. Identification of markers at various stages of batch fermentation and improved production of xylanase using Aspergillus niger (KP874102.1). 3 Biotech 8, 337 (2018). https://doi.org/10.1007/s13205-018-1363-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1363-3