Abstract
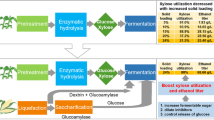
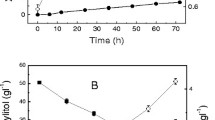
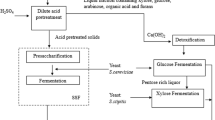
The efficient utilization of lignocellulosic biomass for ethanol production depends on the fermentability of the biomass hydrolysate obtained after pretreatment. In this work we evaluated the kinetics of ethanol production from xylose using Pichia stipitis in acid-treated corn cob hydrolysate. Acetic acid is one of the main inhibitors in corn cob hydrolysate that negatively impacts kinetics of xylose fermentation by P. stipitis. Unstructured kinetic model has been formulated that describes cell mass growth and ethanol production as a function of xylose, oxygen, ethanol, and acetic acid concentration. Kinetic parameters were estimated under different operating conditions affecting xylose fermentation. This is the first report on kinetics of xylose fermentation by P. stipitis which includes inhibition of acetic acid on growth and product formation. In the presence of acetic acid in the hydrolysate, the model accurately predicted reduction in maximum specific growth rate (from 0.23 to 0.15 h−1) and increase in ethanol yield per unit biomass (from 3 to 6.2 gg−1), which was also observed during experimental trials. Presence of acetic acid in the fermentation led to significant reduction in the cell growth rate, reduction in xylose consumption and ethanol production rate. The developed model accurately described physiological state of P. stipitis during corn cob hydrolysate fermentation. Proposed model can be used to predict the influence of xylose, ethanol, oxygen, and acetic acid concentration on cell growth and ethanol productivity in industrial fermentation.




Similar content being viewed by others
Abbreviations
- dp′:
-
Average of experimental values
- dp:
-
Experimental value
- Xp:
-
Value predicted by mathematical model
- np:
-
Number of experiment points
- μ :
-
Specific growth rate (h−1)
- q s :
-
Specific substrate uptake rate (h−1)
- q p :
-
Specific ethanol production rate (h−1)
- C ox :
-
Dissolved oxygen concentration (mg L−1)
- X :
-
Cell mass concentration (g L−1)
- μ max :
-
Maximum specific growth rate (h−1)
- S :
-
Xylose concentration (g L−1)
- K S :
-
Saturation constant governing xylose-limited growth (g L−1)
- K I :
-
Substrate inhibition constant for growth (g L−1)
- P :
-
Ethanol concentration (g L−1)
- n :
-
Exponents governing ethanol inhibition of growth
- P max :
-
Maximum ethanol concentration allowing growth (g L−1)
- K ox :
-
Saturation constant for oxygen-limited growth (mg L−1)
- C A :
-
Acetic acid concentration (g L−1)
- C Amax :
-
Maximum concentration of acetic acid at which cell growth ceases (g L−1)
- m :
-
Parameter used to describe acetic acid inhibition
- Y x/s :
-
Biomass yield (g g−1)
- m S :
-
Maintenance coefficient (g g−1 h−1)
- Y p/x :
-
Yield of ethanol per biomass formed (g g−1)
- K La :
-
Volumetric mass transfer coefficient (s−1)
- C ox*:
-
Critical dissolved oxygen concentration (mg L−1)
- Y x/ox :
-
Yield of biomass per oxygen consumed (g mg−1)
- h :
-
Time (h)
References
Abbott DA, Ingledew WM (2004) Buffering capacity of whole corn mash alters concentrations of organic acids required to inhibit growth of Saccharomyces cerevisiae and ethanol production. Biotech Lett 26:1313–1316
Agbogbo FK, Coward-Kelly G, Torry-Smith M, Wenger KS (2006) Fermentation of glucose/xylose mixtures using Pichia stipitis. Process Biochem 41(11):2333–2336
Andrade RR, Rivera EC, Costa A, Atala DIP, Maugeri F (2007) Estimation of temperature dependent parameters of a batch alcoholic fermentation process. Appl Biochem Biotechnol 136(140):753–763
Andrade RR, Rabelo SC, Filho RM, Costa A (2012) Evaluation of the alcoholic fermentation kinetics of enzymatic hydrolysates from sugarcane bagasse (Saccharum officinarum L.). J Chem Technol Biotechnol. doi:10.1002/jctb.3937
Bellissimi E, van Dijken JP, Pronk JT, van Maris AJA (2009) Effect of acetic acid on the kinetics of xylose fermentation by an engineered, xylose-isomerase-based Saccharomyces cerevisiae strain. FEMS Yeast Res 9:358–364
Casey E, Sedlak M, Nancy WY, Mosier NS (2010) Effect of acetic acid and pH on cofermentation of glucose and xylose to ethanol by a genetically engineered strain of Saccharomyces cerevisiae. FEMS Yeast Res 10:385–393
Farias D, Andrade RR, Maugeri F (2014) Kinetic modeling of ethanol production by Scheffersomyces stipitis from xylose. Appl Biochem Biotechnol 172:361–379
Graves T, Narendranath NV, Dawson K, Power R (2000) Effect of pH and lactic or acetic acid on ethanol productivity by Saccharomyces cerevisiae in corn mash. J Ind Microbiol Biotechnol 33(6):469–474
Hahn-Hagerdal B, Jeppsson H, Skoog K, Prior BA (1994) Biochemistry and physiology of xylose fermentation by yests. Enzyme Microb Technol 16:933–943
Heer D, Sauer U (2008) Identification of furfural as a key toxin in lignocellulosic hydrolysates and evolution of a tolerant yeast strain. Microb Biotechnol 1:497–506
Maiorella B, Blanch HW, Wilke CR (1983) By-product inhibition effects on ethanolic fermentation by Saccharomyces cerevisiae. Biotechnol Bioeng 25:103–121
Narendranath NV, Thomas KC, Ingledew WM (2001) Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J Ind Microbiol Biotechnol 26:171–177
Nichols NN, Sharma LN, Mowery RA, Chambliss CK, van Walsum GP, Dien BS, Lten LB (2008) Fungal metabolism of fermentation inhibitors present in corn stover dilute acid hydrolysate. Enzyme Microb Technol 42:624–630
Palmqvist E, Hahn-Hagerdal B (2000) Fermentation of lignocellulosic hydrolysates. I: inhibition and detoxification. Bioresour Technol 74:17–24
Pampulha ME, Loureiro V (1989) Interaction of the effects of acetic acid and ethanol on inhibition of fermentation in Saccharomyces cerevisiae. Biotechnol Lett 11:269–274
Pampulha ME, Loureiro-Das MC (2000) Energetics of the effect of acetic acid on growth of Saccharomyces cerevisiae. FEMS Microbiol Lett 184(1):69–72
Rivera EC, Costa A, Atala DIP, Maugeri F, Maciel RW, Filho RM (2006) Evaluation of optimization techniques for parameter estimation: application to ethanol fermentation considering the effect of temperature. Process Biochem 41:1682–1687
Sensoz S, Angin D, Yorgun S (2000) Influence of particle size on the pyrolysis of rapeseed (Brassica napus L.): fuel properties of bio-oil. Biomass Bioenergy 19:271–279
Slininger PJ, Dien BS, Lomont JM, Bothast RJ, Ladisch MR, Okos MR (2014) Evaluation of kinetic model for computer simulation of growth and fermentation by Scheffersomyces (Pichia) stipitis fed d-xylose. Biotechnol Bioeng 111(8):1532–1540
Sluiter J, Sluiter A (2011) Laboratory analytical procedure (LAP) review and integration. https://www.nrel.gov/bioenergy/biomass-compositional-analysis.html. Accessed 15 Oct 2015
Taherzadeh MJ, Niklasson C, Liden G (1997) Acetic acid—friend or foe in anaerobic batch conversion of glucose to ethanol by Saccharomyces cerevisiae. Chem Eng Sci 52:2653–2659
Thomas KC, Hynes SH, Ingledew WM (2002) Influence of medium buffering capacity on inhibition of Saccharomyces cerevisiae growth by acetic acid and lactic acids. Appl Environ Microb 68:1616–1623
Wang Y, Liu S (2014) Kinetic modeling of ethanol batch fermentation by Escherichia coli FBWHR using hot-water sugar maple wood extract hydrolyzate as substrate. Energies 7:8411–8426
Wright L (2006) Worldwide commercial development of bioenergy with a focus on energy crop-based projects. Biomass Bioenergy 30:706–714
Acknowledgements
The authors gratefully acknowledge Praj industries Ltd. Pune for financial support to conduct the research work. The authors are also thankful to analytical team of Praj Matrix—R&D Center for providing assistance with analytical measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing or conflict of interest.
Rights and permissions
About this article
Cite this article
Kashid, M., Ghosalkar, A. Evaluation of fermentation kinetics of acid-treated corn cob hydrolysate for xylose fermentation in the presence of acetic acid by Pichia stipitis . 3 Biotech 7, 240 (2017). https://doi.org/10.1007/s13205-017-0873-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0873-8