Abstract
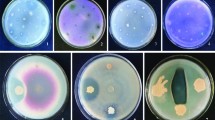
Beneficial aspects of endophytic microorganisms have motivated researchers to explore plant endophytic world. The present study was aimed to isolate and characterize the seed-borne endophytic bacteria from diverse maize genotypes. Eighty maize seed endophytic bacteria (MSEB), isolated from 30 maize genotypes, were characterized using polyphasic approach. The dendrograms and phylogenetic tree generated on the basis of ARDRA analysis and metabolic profiling of endophytic bacteria revealed genotypic and biochemical diversity among MSEB. The 16S rDNA sequence analysis revealed Bacillus as the most dominant encountered genus affiliated with Phylum Firmicutes. Few isolates belonged to genus Staphylococcus, whereas one isolate was identified as Corynebacterium sp. under Phylum Actinobacteria. Majority of the MSEB isolates exhibited antagonism against phytopathogenic fungi, production of ammonia, and secretion of lytic enzymes; some isolates also exhibited indole acetic acid production, the traits of which can be helpful in endophytic establishment and advantageous to the host plant. Besides, many MSEB exhibited tolerance to salinity (10%), osmotic stress (40% PEG6000), and temperature (60 °C), indicating their possible application under stress conditions. Endophytic nature of the selected MSEB isolates was confirmed by tracking their presence in shoots, leaves, and roots of the host seedlings with the help of biochemical marker (rifampicin resistance). Thus, the MSEB identified in the present study can be explored as potential bioinputs for improving plant growth and productivity under stressed conditions, besides helping in understanding the plant–endophyte interactions.



Similar content being viewed by others
References
Akram, Shahid M, Tariq M, Azeem M, Javed MT, Saleem S, Riaz S (2016) Deciphering Staphylococcus sciuri SAT-17 mediated anti-oxidative defense mechanisms and growth modulations in salt stressed maize (Zea mays L.). Front Microbiol 7(867):1–14
Azevedo JL, Maccheroni W Jr, Pereira JO, Araújo WL (2000) Endophytic microorganisms: a review on insect control and recent advances on tropical plants. Electron J Biotechnol 3:e1–e4
Bacon CW, White JF (2000) Microb Endophytes. Marcel Deker Inc, New York
Bacon CW, Palencia ER, Hinton DM (2015) Abiotic and biotic plant stress-tolerant and beneficial secondary metabolites produced by endophytic Bacillus species. In: Arora NK (ed) Plant microbes symbiosis: applied facets. Springer, New Delhi, pp 163–177. doi: 10.1007/978-81-322-2068-8_8
Bent E, Chanway CP (1998) The growth-promoting effects of a bacterial endophyte on lodgepole pine are partially inhibited by the presence of other rhizobacteria. Can J Microbiol 44:980–988
Berg G, Krechel A, Ditz M, Sikora R, Ulrich A, Hallmann J (2005) Endophytic and ectophytic potato-associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi. FEMS Microbiol Ecol 51:215–229
Bhatt HB and Singh SP (2016) Phylogenetic and phenogram based diversity of haloalkaliphilic bacteria from the saline desert. In: Bhukya B, Tangutur AD (ed) Microbial biotechnology-technological challenges and developmental trends. Apple Academic Press pp 373–386 doi:10.1201/b19978-24
Borriss R (2011) Use of plant-associated Bacillus strains as biofertilizers and biocontrol agents in agriculture. In: Maheshwari DK (ed) Bacteria in agrobiology: Plant growth responses. Springer Berlin Heidelberg. doi:10.1007/978-3-642-20332-9_3
Brick JM, Bostock RM, Silversone SE (1991) Rapid in situ assay for indole acetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol 57:535–538
Chee-Sanford JC, Williams MM, Davis AS, Sims GK (2006) Do microorganisms influence seed-bank dynamics? Weed Sci 54:575–587
Chen WP, Kuo TT (1993) A simple and rapid method for the preparation of Gram-negative bacterial genomic DNA. Nucl Ac Res 21:2260
Dye DW (1962) The inadequacy of the usual determinative tests for identification of Xanthomonas spp. NZT Sci 5:393–416
Emmyrafedziawati AKR, Stella M (2015) Hydrolysis of carboxymethyl cellulose (CMC) by Bacillus isolated from compost. J Trop Agric and Fd Sc 43(2):129–135
Figueiredo JEF, Gomes EA, Guimarães CT, Lana UGP, Teixeira MA, Lima GVC, Bressan W (2009) Molecular analysis of endophytic bacteria from the genus Bacillus isolated from tropical maize (Zea mays L.). Braz J Microbiol 40:522–534
Glandorf DCM, Brand I, Bakker PAHM, Schippers B (1992) Stability of rifampicin resistance as a marker for root colonization studies of Pseudomonas putida in the field. Plant Soil 147:135–142
Guedes HV, dos Santos ST, Perin L, Teixeira KR, Reis VM, Baldani JI (2008) Polyphasic characterization of Gluconacetobacter diazotrophicus isolates obtained from different sugarcane varieties. Braz J Microbiol 39(4):718–723
Hankin L, Zucker M, Sands DC (1971) Improved solid medium for the detection and enumeration of pectolytic bacteria. Appl Microbiol 22:205–209
Hardoim PR, von Overbeek LS, von Elsas JD (2008) Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol 16:463–471
Heyndrickx M, Vauterin L, Vandamme P, Kersters K, De Vos P (1996) Applicability of combined amplified ribosomal DNA restriction analysis (ARDRA) patterns in bacterial phylogeny and taxonomy. J Microbiol Methods 26:247–259
Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST (1994) Bergy’s manual of determinative bacteriology. Williams & Wikins Press, Baltimore
Hu XF, Chen J, Guo JF (2006) Two phosphate and potassium solubilizing bacteria isolated from Tiannu mountain, Zhejiang, China. World J Microbiol Biotechnol 22:983–990
James EK, Gyaneshwar P, Mathan N, Barraquio WL, Reddy PM, Iannetta PP, Olivares FL, Ladha JK (2002) Infection and colonization of rice seedlings by the plant growth-promoting bacterium Herbaspirillum seropedicae Z67. Mol Plant Microbe Interact 15:894–906
Jha PN, Kumar A (2007) Endophytic colonization of Typha australis by a plant growth promoting bacterium Klebsiella oxytoca GR 3. J Appl Microbiol 103:1311–1320
Johnston-Monje D, Raizada MN (2011) Conservation and diversity of seed associated endophytes in Zea across boundaries of evolution, ethnography and ecology. PLoS One 6(6):1–22
Khalaf EM, Raizada MN (2016) Taxonomic and functional diversity of cultured seed associated microbes of the cucurbit family. BMC Microbiol 16:1–16
Laguerre G, Allard MR, Revoy F, Amarger N (1994) Rapid identification of rhizobia by restriction fragment length polymorphism analysis of PCR amplified 16S rRNA genes. Appl Environ Microbiol 60:56–63
Malinowski DP, Belesky DP (2000) Adaptations of endophyte infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci 40:923–940
Mano H, Tanaka F, Watanabe A, Kaga H, Okunishi S, Morisaki H (2006) Culturable surface and endophytic bacterial flora of the maturing seeds of rice plants (Oryza sativa) cultivated in a paddy field. Microbes Environ 21:86–100
Marques APGC, Pires C, Moreira H, Rangel AOSS, Castro PML (2009) Assessment of the plant growth promotion abilities of six bacterial isolates using Zea mays as indicator plant. Soil Biol Biochem 42:1229–1235
Nelson EB (2004) Microbial dynamics and interactions in the spermosphere. Ann Rev Phytopathol 42:271–309
Parihar CM, Jat SL, Singh AK, Kumar RS, Hooda KS, Chikkappa GK, Singh DK (2011) Maize production technologies in India. DMR Technical bulletin, Directorate of maize research, Pusa Campus, New Delhi, p 30
Passari AK, Mishra VK, Gupta VK, Yadav MK, Saikia R, Singh BP (2015) In vitro and in vivo plant growth promoting activities and DNA fingerprinting of antagonistic endophytic actinomycetes associated with medicinal plants. PLoS One 10(9):1–18
Pikovskaya RI (1948) Mobilization of phosphorus and soil in connection with the vital activity of some microbial species. Mikrobiologia 17:362–370
Plou FJ, Ferrer M, Nuero OM, Calvo MV, Alcalde M, Reyes F, Ballesteros A (1998) analysis of tween 80 as an esterase/lipase substrate for lipolytic activity assay. Biotechnol Tech 12:183–186
Prasad MP, Darar S (2014) Identification and characterization of endophytic bacteria from fruits like avacado and black grapes. Int J Curr Microbiol App Sci 3(8):937–947
Rohlf FJ (1988) NTSYSpc Numerical taxonomy and multivariate analysis system. Exeter, Exeter
Sahu MK, Sivakumar K, Kannan L (2005) Degradation of organic matters by the extracellular enzymes of actinomycetes isolated from the sediments and mollusks of the vellar estuary. J Aquat Biol 20:142–144
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual, 2nd edn. Cold Spring Harbor laboratory, NewYork
Sanjay A, Purvi NP, Meghna JV, Rao GG (2014) Isolation and characterization of endophytic bacteria colonizing halophyte and other salt tolerant plant species from coastal Gujarat. Afr J Microbiol Res 8(17):1779–1788
Saravanan VS, Subramoniam SR, Raj SA (2004) Assessing in vitro solubilization potential of different zinc solubilizing bacterial (ZSB) isolates. Brazilian J Microbiol 35(1–2):121–125
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophore. Anal Biochem 160:47–56
Senthilkumar M, Govindasamy V, Annapurna K (2007) Role of antibiosis in suppression of charcoal rot disease by soybean endophyte Paenibacillus sp. HKA 15. Curr Microbiol 55:25–29
Smibert RM, Krieg NR (1994) Phenotypic characterization. In: Gerhardt P, Murray RGE, Wood WA, Krieg NR (eds) Methods for general and molecular bacteriology. ASM, Washington, pp 607–654
Strobel G, Daisy B, Castillo U, Harper J (2004) Natural products from endophytic microorganisms. J Nat Prod 67:257–268
Sturz AV, Christie BR, Nowak J (2000) Bacterial endophytes: potential role in developing sustainable system of crop production. Crit Rev Plant Sci 19:1–30
Sullivan TJ, Rodstrom J, Vandop J, Librizzi J, Graham C, Schardl CL, Bultman TL (2007) Symbiont mediated change in Lolium arundinaceum inducible defenses: evidence from changes in gene expression and leaf composition. New Phytol 176:673–679
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Truyens S, Weyens N, Cuypers A, Vangronsveld J (2015) Bacterial seed endophytes: genera, vertical transmission and interaction with plants. Environ Microbiol Rep 7(1):40–50
Viti C, Giovannett L (2005) Characterization of cultivable heterotrophic bacterial communities in Cr-polluted and unpolluted soils using Biolog and ARDRA approaches. Appl Soil Ecol 28:101–112
West CP, Izekor E, Turner KE, Elmi AA (1993) Endophyte effects on growth and persistence of tall fescue along a water supply gradient. Agron J 85:264–270
White JF, Torres MS, Sullivan RF, Jabbour RE, Chen Q, Tadych M, Irizarry I, Bergen MS, Havkin-Frenkel D, Belanger FC (2014) Occurrence of Bacillus amyloliquefaciens as a systemic endophyte of vanilla orchids. Microsc Res Techniq 77(11):874–885
Acknowledgements
Authors of this manuscript are thankful to ICAR-AMAAS (Application of Microorganisms in Agriculture and Allied Sectors) for the financial support. Authors are also thankful to the National Coordinator, ICAR-NICRA (National Initiative on Climate Resilient Agriculture) for providing the maize seeds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13205_2017_860_MOESM2_ESM.tif
Figure 5. Dendrogram of MSEB generated on the basis of 16S rDNA restriction profile (EcoRI) by using NTSYSpc (TIFF 842 kb)
13205_2017_860_MOESM3_ESM.tif
Figure 6. Dendrogram of MSEB generated on the basis of 16S rDNA restriction profile (MspI) by using NTSYSpc (TIFF 842 kb)
13205_2017_860_MOESM4_ESM.tif
Figure 7. Dendrogram of MSEB generated on the basis of 16S rDNA restriction profile (Hae III) by using NTSYSpc (TIFF 894 kb)
Rights and permissions
About this article
Cite this article
Bodhankar, S., Grover, M., Hemanth, S. et al. Maize seed endophytic bacteria: dominance of antagonistic, lytic enzyme-producing Bacillus spp.. 3 Biotech 7, 232 (2017). https://doi.org/10.1007/s13205-017-0860-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0860-0