Abstract
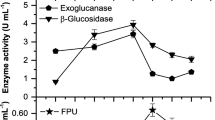
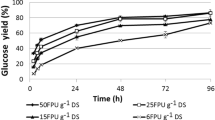
Nocardiopsis sp. KNU was found to degrade various lignocellulosic waste materials, namely, sorghum husk, sugarcane tops and leaves, wheat straw, and rice husk very efficiently. The strain was found to produce high amounts of cellulase and hemicellulase. Augmentation of cotton seed cake as an organic nitrogen source revealed inductions in activities of endoglucanase, glucoamylase, and xylanase up to 70.03, 447.89, and 275.10 U/ml, respectively. Nonionic surfactant Tween-80 addition was found to enhance the activity of endoglucanase enzyme. Cellulase produced by Nocardiopsis sp. KNU utilizing sorghum husk as a substrate was found to retain its stability in various surfactants up to 90%. The produced enzyme was further tested for saccharification of mild alkali pretreated rice husk. The changes in morphology and functional group were analyzed using scanning electron microscopy and Fourier transform infrared spectroscopy. Enzymatic saccharification confirmed the hydrolytic potential of crude cellulase. The hydrolysate products were analyzed by high-performance thin layer chromatography.





Similar content being viewed by others
References
Cheng KK, Cai BY, Zhang JA, Ling HZ, Zhou YJ, Ge JP, Xu JM (2008) Sugarcane bagasse hemicellulose hydrolysate for ethanol production by acid recovery process. Biochem Eng J 38(1):105–109. doi:10.1016/j.bej.2007.07.012
Chiranjeevi T, Baby Rani G, Chandel AK, Satish Sekhar PV, Prakasham RS, Addepally U (2012) Optimization of holocellulolytic enzymes production by Cladosporium cladosporioides using Taguchi-L’16 orthogonal array. J Biobased Mater Bio. doi:10.1166/jbmb.2012.1201
Coman G, Bahrim G (2011) Optimization of xylanase production by Streptomyces sp. P12-137 using response surface methodology and central composite design. Ann Microbiol 61(4):773–779. doi:10.1007/s13213-010-0195-0
de Lima ALG, do Nascimento RP, da Silva Bon EP, Coelho RRR (2005) Streptomyces drozdowiczii cellulase production using agro-industrial by-products and its potential use in the detergent and textile industries. Enzyme Microb Technol 37:272–277. doi:10.1016/j.enzmictec.2005.03.016
Engelhardt K, Degnes KF, Kemmler M, Bredholt H, Fjaervik E, Klinkenberg G, Sletta H, Ellingsen TE, Zotchev SB (2010) Production of a new thiopeptide antibiotic, TP-1161, by a marine Nocardiopsis species. Appl Environ Microbiol 76(15):4969–4976. doi:10.1128/AEM.00741-10
Eriksson T, Börjesson J, Tjerneld F (2002) Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzyme Microb Technol 31(3):353–364. doi:10.1016/s0141-0229(02)00134-5
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59(2):257–268. doi:10.1351/pac198759020257
Goluguri BR, Thulluri C, Addepally U, Shetty PR (2016) Novel alkali-thermostable xylanase from Thielaviopsis basicola (MTCC 1467): purification and kinetic characterization. Int J Biol Macromol 82:823–829. doi:10.1016/j.ijbiomac.2015.10.055
He YF, Pang YZ, Liu YP, Li XJ, Wang KS (2008) Physicochemical characterization of rice straw pretreated with sodium hydroxide in the solid state for enhancing biogas production. Energy Fuels 22(4):2775–2781. doi:10.1021/ef8000967
Kamini NR, Mala JGS, Puvanakrishnan R (1998) Lipase production from Aspergillus niger by solid-state fermentation using gingelly oil cake. Process Biochem 33(5):505–511. doi:10.1016/s0032-9592(98)00005-3
Kshirsagar SD, Waghmare PR, Loni PC, Patil SA, Govindwar SP (2015) Dilute acid pretreatment of rice straw, structural characterization and optimization of enzymatic hydrolysis conditions by response surface methodology. RSC Adv 5(58):46525–46533. doi:10.1039/c5ra04430h
Kumar S, Satyanarayana T (2004) Statistical optimization of a thermostable and neutral glucoamylase production by a thermophilic mold Thermomucor indicae-seudaticae in solid-state fermentation. World J Microbiol Biotechnol 20(9):895–902. doi:10.1007/s11274-004-2891-z
Liang YL, Zhang Z, Wu M, Wu Y, Feng JX (2014) Isolation, screening, and identification of cellulolytic bacteria from natural reserves in the subtropical region of China and optimization of cellulase production by Paenibacillus terrae ME27-1. Biomed Res Int 2014:512497. doi:10.1155/2014/512497
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577. doi:10.1128/MMBR.66.3.506-577.2002
Miller GL (1959) Use of dinitrosalicyclic reagent for determination of reducing sugar. Anal Chem 31:426–428. doi:10.1021/ac60147a030
Nathan VK, Rani ME, Rathinasamy G, Dhiraviam KN, Jayavel S (2014) Process optimization and production kinetics for cellulase production by Trichoderma viride VKF3. Springerplus 3:92. doi:10.1186/2193-1801-3-92
Nelson ML, O’Connor RT (1964) Relation of certain infrared bands to cellulose crystallinity and crystal latticed type. Part I. Spectra of lattice types I, II, III and of amorphous cellulose. J Appl Poly Sci 8:1311–1324. doi:10.1002/app.1964.070080322
Nitisinprasert S, Temmes A (1991) The characteristics of a new non-spore-forming cellulolytic mesophilic anaerobe strain CM126 isolated from municipal sewage sludge. J Appl Bacteriol 71:154–161
Okino S, Ikeo M, Ueno Y, Taneda D (2013) Effects of Tween 80 on cellulase stability under agitated conditions. Bioresour Technol 142:535–539. doi:10.1016/j.biortech.2013.05.078
Panagiotopoulos IA, Pasias S, Bakker RR, de Vrije T, Papayannakos N, Claassen PA, Koukios EG (2013) Biodiesel and biohydrogen production from cotton-seed cake in a biorefinery concept. Bioresour Technol 136:78–86. doi:10.1016/j.biortech.2013.02.061
Pardo AG (1996) Effect of surfactants on cellulase production by Nectria catalinensis. Curr Microbiol 33(4):275–278. doi:10.1007/s002849900113
Pathak AP, Deshmukh KB (2012) Alkaline protease production, extraction and characterization from alkaliphilic Bacillus licheniformis KBDL4: a Lonar soda lake isolate. Indian J Exp Biol 50(8):569–576
Rawat R, Srivastava N, Chadha BS, Oberoi HS (2014) Generating fermentable sugars from rice straw using functionally active cellulolytic enzymes from Aspergillus niger HO. Energy Fuels 28(8):5067–5075. doi:10.1021/ef500891g
Reese ET, Maguire A (1969) Surfactants as stimulants of enzyme production by microorganisms. Appl Microbiol 17(2):242–245
Rocha VAL, Maeda RN, Anna LMMS, Pereira N (2013) Sugarcane bagasse as feedstock for cellulase production by Trichoderma harzianum in optimized culture medium. Electron J Biotechnol. doi:10.2225/vol16issue5fulltext1
Rodhe AV, Sateesh L, Sridevi J, Venkateswarlu B, Venkateswar Rao L (2011) Enzymatic hydrolysis of sorghum straw using native cellulase produced by T. reesei NCIM 992 under solid state fermentation using rice straw. 3 Biotech 1(4):207–215. doi:10.1007/s13205-011-0024-6
Rubin EM (2008) Genomics of cellulosic biofuels. Nature 454(7206):841–845. doi:10.1038/nature07190
Saratale GD, Oh M-K (2015) Characterization of poly-3-hydroxybutyrate (PHB) produced from Ralstonia eutropha using an alkali-pretreated biomass feedstock. Int J Biol Macromol 80:627–635. doi:10.1016/j.ijbiomac.2015.07.034
Saratale GD, Saratale RG, Lo YC, Chang JS (2010) Multicomponent cellulase production by Cellulomonas biazotea NCIM-2550 and its applications for cellulosic biohydrogen production. Biotechnol Prog 26(2):406–416. doi:10.1002/btpr.342
Saratale GD, Saratale RG, Oh SE (2012) Production and characterization of multiple cellulolytic enzymes by isolated Streptomyces sp. MDS. Biomass Bioenergy 47:302–315. doi:10.1016/j.biombioe.2012.09.030
Sekurova O, Hv Sletta, Ellingsen TE, Valla S, Zotchev S (1999) Molecular cloning and analysis of a pleiotropic regulatory gene locus from the nystatin producer Streptomyces noursei ATCC11455. FEMS Microbiol Lett 177(2):297–304. doi:10.1111/j.1574-6968.1999.tb13746.x
Stamford TLM, Stamford NP, Coelho LCBB, Araújo JM (2001) Production and characterization of a thermostable α-amylase from Nocardiopsis sp. endophyte of yam bean. Bioresour Technol 76(2):137–141. doi:10.1016/s0960-8524(00)00089-4
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11. doi:10.1016/S0960-8524(01)00212-7
Sun JX, Sun XF, Zhao H, Sun RC (2004) Isolation and characterization of cellulose from sugarcane bagasse. Polym Degrad Stab 84(2):331–339. doi:10.1016/j.polymdegradstab.2004.02.008
Swain RR, Dekker EE (1966) Seed germination studies. I. Purification and properties of an alpha-amylase from the cotyledons of germinating peas. Biochim Biophys Acta 122(1):75–86
Tsujibo H, Kubota T, Yamamoto M, Miyamoto K, Inamori Y (2003) Characterization of chitinase genes from an alkaliphilic actinomycete, Nocardiopsis prasina OPC-131. Appl Environ Microbiol 69(2):894–900. doi:10.1128/aem.69.2.894-900.2003
Waghmare PR, Kadam AA, Saratale GD, Govindwar SP (2014a) Enzymatic hydrolysis and characterization of waste lignocellulosic biomass produced after dye bioremediation under solid state fermentation. Bioresour Technol 168:136–141. doi:10.1016/j.biortech.2014.02.099
Waghmare PR, Kshirsagar SD, Saratale RG, Govindwar SP, Saratale GD (2014b) Production and characterization of cellulolytic enzymes by isolated Klebsiella sp. PRW-1 using agricultural waste biomass. Emir J Food Agric 26(1):44–59. doi:10.9755/ejfa.v26i1.15296
Walia A, Mehta P, Chauhan A, Kulshrestha S, Shirkot CK (2014) Purification and characterization of cellulase-free low molecular weight endo beta-1,4 xylanase from an alkalophilic Cellulosimicrobium cellulans CKMX1 isolated from mushroom compost. World J Microbiol Biotechnol 30(10):2597–2608. doi:10.1007/s11274-014-1683-3
Walia A, Mehta P, Guleria S, Shirkot CK (2015a) Improvement for enhanced xylanase production by Cellulosimicrobium cellulans CKMX1 using central composite design of response surface methodology. 3 Biotech 5(6):1053–1066. doi:10.1007/s13205-015-0309-2
Walia A, Mehta P, Guleria S, Shirkot CK (2015b) Modification in the properties of paper by using cellulase-free xylanase produced from alkalophilic Cellulosimicrobium cellulans CKMX1 in biobleaching of wheat straw pulp. Can J Microbiol 61(9):671–681. doi:10.1139/cjm-2015-0178
Wang B, Wang X, Feng H (2010) Deconstructing recalcitrant Miscanthus with alkaline peroxide and electrolyzed water. Bioresour Technol 101(2):752–760. doi:10.1016/j.biortech.2009.08.063
Wiselogel A, Tyson J, Johnsson D (1996) Biomass feedstock resources and composition. In: Wyman CE (ed) Handbook of bioethanol: production and utilization. Applied energy technology series. CRC, Tayler & Francis Group, Washington, DC, pp 105–118
Yang H, Wu H, Wang X, Cui Z, Li Y (2011) Selection and characteristics of a switchgrass-colonizing microbial community to produce extracellular cellulases and xylanases. Bioresour Technol 102:3546–3550. doi:10.1016/j.biortech.2010.09.009
Acknowledgements
Siddheshwar D. Kshirsagar would like to thank UGC (University Grants Commission), New Delhi for providing UGC-JRF fellowship under UGC Major Research project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kshirsagar, S.D., Bhalkar, B.N., Waghmare, P.R. et al. Sorghum husk biomass as a potential substrate for production of cellulolytic and xylanolytic enzymes by Nocardiopsis sp. KNU. 3 Biotech 7, 163 (2017). https://doi.org/10.1007/s13205-017-0800-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0800-z