Abstract
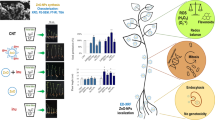
Zein nanoparticles (ZNPs) were synthesized with a cationic surfactant, didodecyldimethylammonium bromide (122.9 ± 0.8 nm, + 59.7 ± 4.4 mV) and a non-ionic surfactant, Tween 80 (118.7 ± 1.7 nm, + 26.4 ± 1.1 mV). Lignin-graft-poly(lactic-co-glycolic) acid nanoparticles (LNPs) were made without surfactants (52.9 ± 0.2 nm, − 54.9 ± 0.5 mV). Both samples were applied as antifungal seed treatments on soybeans, and their impact on germination and plant health was assessed. Treated seeds showed high germination rates (> 90% for all treatment groups), similar to the control group (100%). Root and stem lengths and the dry biomass of treated seeds were not statistically distinguishable from the control. Foliage from seed-treated plants was fed to larvae of Chrysodeixis includens with no differences in mortality between treatments. No translocation of fluorescently tagged particles was observed with fluorescence microscopy following seed treatment and germination. Nano-delivered azoxystrobin provided ~ 100% protection when LNPs were used. Results suggest ZNPs and LNPs are safe and effective delivery systems of active compounds for seed treatments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymeric nanoparticles (PNPs) are proposed as suitable delivery systems in a multitude of agricultural applications (Nuruzzaman et al. 2016; Pascoli et al. 2018), such as for the delivery of herbicides (Tong et al. 2017), insecticides (Gabriel Paulraj et al. 2017; Lichtenberg et al. 2020), fungicides (Kumar et al. 2017; Yang et al. 2014), and plant growth regulators (Pereira et al. 2017). Nanodelivery of agrochemicals is advantageous over conventional applications (Yang et al. 2014), offering protection to the active compound against degradation, increased solubility, improved efficacy (Pereira et al. 2017), controlled release, decreased toxicity of the agrochemical to non-target organisms (Pascoli et al. 2018), and a low negative impact on soil microbiome (Ur Rahim et al. 2021). Some PNPs provide the additional benefit of biodegradability over other types of nanodelivery systems (Kacsó et al. 2018).
The plant’s mechanism of nanoparticle adsorption and absorption to enhance the efficacy of the entrapped agrochemicals is a popular topic of interest (Avellan et al. 2021; Grillo et al. 2021a). Previous studies have shown that these mechanisms are influenced by the particle’s size (Cui et al. 2008), charge (Hu et al. 2020), and hydrophobicity (Sharma et al. 2020; Zhang et al. 2021). Research focusing on the foliar applications of pesticide entrapped nanoparticles is often conducted as these plant tissues are frequently exposed to pests and environmental conditions. For example, Takeshita et al. found that when performing a foliar application of atrazine entrapped PCL nanoparticles on the Florida broad lead mustard, atrazine absorption and translocation were higher for the nanoentrapped compound in comparison to the free atrazine (Takeshita et al. 2021). This effect was attributed to the negative charge of the nanoparticles preventing electrostatic interactions with the cell walls, resulting in its distribution through the apoplast and vascular tissues. Other groups have also observed uptake and translocation through root exposure of plants. Tong et al. discovered that metolachlor entrapped mPEG-poly(lactic-co-glycolic) acid (PLGA) NPs improved adherence of a pesticide to the root cell wall, resulting in penetration into the plant following root exposure (Tong et al. 2017).
Studies focused on nanoparticles (NPs) as delivery systems for seed treatment specifically reported the biochemical activity of the entrapped agrochemical (Yang et al. 2014), the biological activity of the formulation (e.g., antifungal, pesticide activity) relevant for the intended purpose (Kumar et al. 2017; Pereira et al. 2017; Saharan et al. 2015) or the effect of pesticides on the environment (Atwood et al. 2018; Campos et al. 2015; Nettles et al. 2016; Smalling et al. 2018). Meanwhile, the effects of non-loaded NPs on seed quality, germination, and plant growth are generally unknown, nor are other ecotoxicological aspects, such as the effect of agrochemicals on non-target species, which have also gained little attention (Grillo et al. 2021b). To our knowledge, no studies have been published on the translocation and impact of zein nanoparticles (ZNPs) and lignin-graft-poly(lactic-co-glycolic) acid (PLGA) nanoparticles (LNPs) on plant development when used as a seed treatment.
To better understand their environmental impact and role in seed protection and soybean plant development, this study focused on tracking PNPs in seeds after treatment and during germination while determining their effect on plant growth indicators, non-target pests (in this case, insects), and antifungal activity. Two PNPs, both ZNP and LNP, were chosen as the nanodelivery system due to their biodegradable nature (Kacsó et al. 2018) and potential in agriculture (de Oliveira et al. 2014; Ristroph et al. 2017). Previous studies have shown an affinity of positively charged ZNPs for soybean roots grown in hydroponic conditions (Ristroph et al. 2017). Negatively charged core–shell LNPs synthesized with no surfactant (Astete et al. 2020) proved safe for soybean plants in hydroponic systems at concentrations up to 2 mg/mL (Salinas et al. 2021).
In this study, positively charged ZNPs engineered with ionic and non-ionic surfactants and surfactant-less, negatively charged LNPs were tested. These types were chosen as it is known that particle adherence and uptake by plants, as well as plant health, is influenced by size, charge, hydrophobicity, and functional groups available on the NPs’ surface as discussed earlier (Nakasato et al. 2017; Torrent et al. 2020). Soybean was chosen as a model plant due to its importance in agriculture and the economy (Goldsmith 2008). To assess the potential of non-target effects, Chrysodeixis includens, an important defoliator of soybeans, was chosen. Antifungal effectiveness of azoxystrobin against Rhizoctonia solani nanodelivered with PNPs as a seed treatment was also investigated. R. solani was chosen for this experiment due to its aggressive nature, capable of causing seed decay, pre- and post-emergence damping-off, as well as hypocotyl and root rot, leading to stand reductions and loss of yield for soybean growers (Ajayi-Oyetunde and Bradley 2017).
Materials and methods
Reagents and instrumentation
All reagents used were of analytical grade purity and purchased as follows: ethanol from Pharmco Products, Inc. (Brookfield, CT, USA), zein powder, PLGA 50:50 (38–54 kDa), acetone, technical grade azoxystrobin, and didodecyldimethylammonium bromide (DMAB), were purchased from MilliporeSigma (St. Louis, MO, USA). A commercial formulation of azoxystrobin, Dynasty®, was purchased from Syngenta (Wilmington, DE, United States). Tween 80, dimethylformamide (DMF), triethylamine, dichloromethane (DCM), and ethyl acetate were purchased from Fischer Scientific (Pittsburgh, PA, USA), fluorescein isothiocyanate (FITC) from Acros Organics (Fair Lawn, NJ, USA), alkaline lignin from TCI Inc. (Portland, OR, USA), and Tissue-Tek® O.C.T.™ (Optimal Cutting Temperature) Compound from Sakura® (Torrence, CA, USA). Solutions were prepared using ultrapure water (18.2 MΩ, Barnstead, NANOpure Diamond®, Thermo Scientific, Waltham, MA, USA). Samples were homogenized through sonication (Branson 3510, Branson, Danbury, CT, USA) and solvent removal was achieved through vacuum concentration at room temperature using Rotavapor R-300 (Buchi Analytical Inc., New Castle, DE, USA). Plant health studies utilized soybean variety Croplan® RX5677 from Land O’Lakes Inc. (Arden Hills, MN, USA), planted in all-purpose Potting Mix with Controlled Release Fertilizer from Louisiana Nursery (Baton Rouge, LA, USA) and grown in an Intellus environmental controller from Percival-Scientific (Perry, IA, USA) for the duration of the experiments. Seeds were cross-sectioned at -25 °C with a Leica Cryostat CM 1850-3-1 (Buffalo Grove, IL, USA) and analyzed with a Nikon Eclipse Ti2 (Nikon, Tokyo, Japan) inverted microscope equipped with sCMOS pco.edge 5.5 camera (pco., Kelheim, Germany).
Fluorescent tagging
Zein was conjugated with FITC (3.796 molecules FITC/molecule of zein, MW = 20 kD) to allow for nanoparticle tracking using a previously described method (Ristroph et al. 2017). Two grams of zein were dissolved in 70 mL DMF within a round bottom flask at room temperature. Afterward, 70 µL of triethylamine and 25 mg of FITC were added under mild stirring at room temperature. The reaction was stopped after 24 h, and the sample was concentrated under high vacuum. The concentrated suspension (around 25 mL) was washed with dichloromethane (DCM) (50 mL), and the fractions were separated. The remaining free dye was removed by repeated washing of the supernatant containing the zein-FITC in DMF with DCM. The final suspension was dried in an oven (40 °C) under high vacuum for 72 h to remove the remaining organic solvents.
The potential hydrolytic cleavage between zein and FITC was previously assessed (Ristroph et al. 2017) and no hydrolytic products were observed, thus providing support for the presence of FITC in ZNPs in further stages of the experiments.
Fluorescent LNPs (FLNPs) were made by entrapping the fluorescent dye FITC in the LNPs following the same protocol used to make non-loaded LNPs, with the difference that 20 mg of FITC was added to the organic phase. Synthesis was followed by a washing step, to eliminate free FITC from the FLNP solution. A volume of 10 mL FLNP solution was placed within a dialysis bag and submersed in 1000 mL DI water for 24 h over stirring. Water was replaced every hour for 8 h to ensure sink conditions.
PNP synthesis and characterization
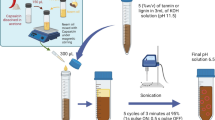
ZNPs were prepared by nanoprecipitation using an adapted version of a previously published synthesis protocol with two surfactants, DMAB and Tween 80 (Kacsó et al. 2018). The organic phase was formed by dissolving 3778 mg of zein in 120 mL of an acetone–water [80:20 (v/v)] solution under magnetic stirring at room temperature. The aqueous phase consisted of 394 mg of the surfactant DMAB dissolved in 682 mL of deionized water at room temperature. Next, the organic phase was added to the aqueous phase under strong stirring at room temperature. The mixture was then passed through a microfluidizer (Microfluidics M-110P, Westwood, MA, USA) 3 times at 25,000 psi. Finally, the acetone was evaporated with a rotary evaporator (Rotavapor R-300 Buchi Analytical Inc., New Castle, DE, USA), under vacuum, and the solution was concentrated until a final volume of 400 mL was obtained. The resultant NP solutions had a final concentration of 10 mg NP/mL. In the case of ZNPs prepared with the surfactant Tween 80, the synthesis was similar, with 1667 mg zein in 47 mL of acetone–water [80:20 (v/v)] solution and 2333 mg Tween 80 in 467 mL of deionized water. For fluorescently tagged particles (FZNP), a 1:1 mixture of zein:zein–FITC was used, where zein–FITC was previously synthesized by covalently attaching FITC to zein.
LNPs were made from a graft lignin-PLGA biopolymer (LGN-g-PLGA) obtained using an acylation reaction as previously described, and the LNPs were synthesized by a modified emulsion evaporation technique (Astete et al. 2020). No surfactants were added in the aqueous phase, and no purification steps were required. Briefly, 150–500 mg of LGN-g-PLGA was dissolved in 5 mL of ethyl acetate at room temperature under vigorous stirring. Next, the organic phase was added to the aqueous phase (50 mL of DI water). After 10 min of mixing, the suspension was passed through a microfluidizer (Microfluidics Corp., Westwood, MA, USA) at 30,000 psi four times at 4 °C for homogenization purposes. Afterward, the organic solvent was evaporated in a rotavapor R-300 (Buchi Corporation, New Castle, DE, USA) at 32 °C under vacuum for at least 45 min until the desired concentration (10 mg/mL) was achieved.
Entrapment of the antifungal, azoxystrobin, was added in the organic phase during NP synthesis in a ratio of 10 mg per 100 mg of polymer. Theoretical azoxystrobin loading was calculated as the amount of total entrapped antifungal divided by the total nanoparticle weight, expressed as a percentage. The fluorescently tagged LNPs were synthesized similarly, except that FITC was entrapped in the particles instead of azoxystrobin. The particles were washed by dialysis for 24 h to remove free FITC prior to using the fluorescently tagged LNPs as a tracking system.
Nanoparticle diameter, polydispersity, and zeta potential of the PNPs were assessed by dynamic light scattering (Zetasizer ZS, Malvern Instruments Ltd.) in DI water. All measurements were carried out at 25 °C using three replicates of 0.5 mg NP/mL, considering a mono-modal distribution. The morphology of azoxystrobin-loaded PNP was determined by transmission electron microscopy (TEM) JEM-1400 (Jeol USA Inc. Peabody, MA) as follows. The PNP suspension was placed as 1 mg/ml droplets on a copper grid with a contrast agent. After removal of the excess sample, the grid was air-dried for 15 min before being placed into the TEM chamber for analysis.
For simplification, ZNPs synthesized with DMAB will be referred to as ZNP-D, those with Tween 80 as ZNP-T, and lignin-PLGA as LNP, followed by the letter A if loaded with azoxystrobin.
Seed treatment
Soybean seeds were treated with the three PNP types by immersion in freshly prepared NP colloids, 1 mL/g of seed for 5 min. Treated seeds were dried overnight on a sieve at room temperature, after which they were stored at 4 °C. Solution of 10 mg PNP/mL of FZNP and FITC loaded LNP were used for PNP tracking. For plant health studies, non-fluorescently tagged particles were used at two concentrations: 5 and 10 mg PNP/mL. High concentrations were chosen to enable better PNP tracking in seeds and assess plant health under the same conditions. Insect mortality (non-target assessment) and antifungal studies were carried out with treated soybean seeds following the same protocol with PNP solution containing azoxystrobin adjusted to a final concentration of 0.5 mg/mL of antifungal, respectively, with 0.5 mg/mL azoxystrobin dissolved in water (A) or the commercial formulation (CA), Dynasty®. Treatment of control seeds followed the same protocol, but seeds were immersed in deionized water.
Seed germination
Seed germination tests were carried out using a modified version of the method published elsewhere (Rao et al. 2006). Soybeans seeds were placed on water-soaked paper towels 2 cm apart, ten seeds per paper towel, rolled up and covered with aluminum foil to maintain constant humidity. Replicates of five were completed for each treatment. Seeds prepared for germination in this way were placed in an incubator at 27 °C and 70% humidity.
Plant health
Seeds, eight per treatment were sown individually in pots, and kept in the incubator at 27 °C and 70% humidity for the emergence study. Plants were collected after 28 days, where the root and stem length and chlorophyll content were measured. Chlorophyll was measured using a SPAD 502-Plus Chlorophyll Meter (Konica Minolta, Tokyo, Japan). Dried biomass of roots and stem was weighed after drying for 72 h at 50 °C.
Particle tracking
Non-treated and treated, dry, non-germinated and 1-day germinated soybean seeds were incorporated in O.C.T. and cut with a cryostat microtome to 35 µm thickness, placed on glass microscope slides, and fixed with a drop of water, if necessary. Three seeds were analyzed for each treatment and stage of growth. The obtained cross-sections were studied under a Nikon Eclipse Ti2 inverted microscope (Nikon, Tokyo, Japan) with a sCMOS pco.edge camera (pco., Kelheim, Germany). For the assessment of NP distribution inside the seed, the camera was fitted with the corresponding filter for FITC (480 nm excitation (30 nm bandwidth) and 535 nm emission (20 nm bandwidth)).
Soybean seed inoculation and growth for antifungal experiment
The experimental unit was one 4-inch circular pot containing SunGro® Professional Growing Mix, Fafard® 3B Mix Metro-Mix® 830 (SunGro Horticulture, Agawam, MA, USA) supplemented with Osmocote, slow-release 14-14-14 fertilizer (Scotts Miracle-Gro, Marysville, OH, USA). Prior to planting a 1-inch-deep furrow was pressed across the pot’s diameter with a specialized steel tool. Seeds were sown at a rate of four seeds per pot, evenly spaced across the length of the furrow. Inoculated treatments (IC) received four seeds sterilized twice and infested with R. solani evenly spaced across the length of the furrow. The difference between soybean seedlings infected with R. solani 14 days after inoculation and planting versus non-inoculated plants are shown in Fig.S1.
After sowing, seeds were immediately covered with potting soil, irrigated briefly overhead, and moved to automatic flood-irrigated greenhouse tables. Supplemental lighting was supplied with LEDs on a 12-h photoperiod. Emergence was counted 3 times. Two weeks after planting, soybean plants were removed from pots and gently washed until free of potting medium. Root and shoot lengths were measured, and the plants exhibiting lesions were counted and expressed as a percentage. Finally, after drying for 10 days, biomass was weighed.
Insect bioassay
Non-target impacts of nine different soybean seed treatments were tested on first instar C. includens (Walker) (Lepidoptera: Noctuidae) through foliar consumption. The C. includens colony used in this experiment, LSU1, was reared in the Entomology Department at Louisiana State University at 26 °C, 50% RH, and 14:10 (L:D) photoperiod on artificial meridic diet (Southland Products Inc., Lake Village, AR, USA) (Bonser et al. 2020). Soybean plants were grown at the LSU AgCenter Plant Material Center Greenhouse under greenhouse conditions (25 ± 5 °C, 50 ± 5% RH, and 14:10 L:D). Soybean seeds were grown in 1.8 L pots (Dillen Products, Middlefield, OH, USA), using Miracle-Gro® Potting Mix (Scotts Miracle-Gro Company, LLC, Marysville, OH, USA) supplemented with Osmocote (15-15-15), slow-release fertilizer. All soybeans tested were at the V4 stage (Fehr and Caviness 1977).
Four soybean seeds of a single treatment were planted per pot and grown to the appropriate stage. Four randomly selected leaves from each pot were cut using a pair of cleaned scissors (3 M, St. Paul, MN, USA) and put into a sterile polystyrene petri dish (100 × 15 mm) (VWR, Radnor, PA, USA) lined with Whatman® 90 mm, Grade 1 filter paper (Sigma-Aldrich, St. Louis, MO, USA). A single C. includens larva was placed onto each leaf using a nylon paintbrush (Royal Brush Manufacturing, Munster, IN, USA). Petri dishes were kept in an I-36VL Percival growth chamber (Percival Scientific, Perry, IA, USA) at 25 °C with 75% RH and 14:10 (L:D) photoperiod. Filter paper was moistened every 2 days with distilled water. After 7 days, mortality was assessed. The experiment was replicated four times, with each plant being treated as a replicate.
Statistical analysis
To assess the effect of non-loaded PNPs on plant development, a single-factor analysis of variance (ANOVA) was used, followed by a two-sample Student’s t test assuming unequal variances using Microsoft Excel® software. A randomized block design analysis was applied to assess antifungal effects of PNPs, using the ARM 2020 software (Gylling Data Management, Brookings, SD, USA). Larval mortality was arcsine square-root transformed for analysis. Normality and homogeneity were assessed with the Shapiro–Wilk test in R 4.0.4 using IDE RStudio (R Core Team 2019; RStudio Team 2020). Transformed mortalities were compared between treatments using a single-factor ANOVA (R Core Team 2019). Means were separated using a post hoc Tukey’s honestly significant difference (HSD) (P < 0.05) (R Core Team 2019).
Results and discussion
Characterization of PNPs
Zein nanoparticles made with DMAB (ZNP-D) presented similar hydrodynamic diameters as ZNPs made with Tween 80 (ZNP-T) and fluorescently tagged ZNPs (FZNP). At the same time, azoxystrobin-loaded ZNPs presented slightly larger diameters than the empty ZNPs, regardless of the surfactant used (ZNP-DA, ZNP-TA) (Table 1). Azoxystrobin theoretical loading was 9.09% for all particle types (Table 1).
Zeta potential was positive for all ZNPs ranging from 20.9 to 59.7 mV. DMAB is a cationic surfactant resulting in a more pronounced positive zeta potential of ZNP-D relative to ZNP-T particles. This difference in charge was also reflected in the NP stability, where over time, a visible aggregation and decrease in zeta potential was noticeable for the ZNP-T particles, but not for the higher charged ZNP-D particles (data not shown). Tween 80 is a non-ionic surfactant. Therefore, zeta potential of ZNP-T is conferred by the intrinsic charge of zein, which may change over time due to a hydrolytic deamidation process resulting in a higher ratio of negatively charged glutamate (Zhang et al. 2011). Lignin-based particles presented the smallest hydrodynamic diameters of 52.9 nm while possessing a zeta potential of -54.9 mV. FITC-loaded LNPs (FLNP) and azoxystrobin-loaded LNPs (LNP-A) resulted in larger particle sizes of 127.8 nm and 178.3 nm, respectively, due to the incorporation of the fluorophore agent and fungicide.
TEM imaging confirmed that azoxystrobin-loaded nanoparticles were spherical in morphology with no visible aggregation (Fig. 1), with similar sizes to those measured via dynamic light scattering (Table 1).
Plant development
Germination rate, emergence rate and root development
All soybean seeds presented signs of germination after the first 24 h, characterized by an increase in size and indications of radicle development. After 5 days of incubation, the germination rate was 90% for all batches and 100% for the control and ZNP-D treatment at 5 mg/mL (Fig. 2A). Total hypocotyl and root length after 5 days of germination was approximately 12 cm for the non-treated control. Hypocotyl and root lengths ranged from 12 cm (for ZNP-treated seeds at 5 mg/mL) to 15 cm (for LNP-treated seeds at 10 mg/mL) and did not differ statistically among treatments (data not shown). The hypocotyl and root length for the LNP treatment can be explained by the numerous phenolic groups available on the lignin structure, which can exert a hormone-like action on young seed biochemical activities, thereby stimulating metabolic processes that improve early development (Buono et al. 2021).
Treatment compared to control, where control is considered 100%. A Germination and emergence; B root length and dry biomass; C stem length and dry biomass; D chlorophyll content of leaves. *Statistically significant difference compared to control following a two-sample t test assuming unequal variances (P < 0.05), where ZNP-D ZNPs synthesized with DMAB, ZNP-T ZNPs synthesized with Tween 80; LNP lignin-PLGA nanoparticles
Similar results have been published for some PNPs when used as seed treatment or germination medium for various seeds. Chitosan NPs (CSNPs) when used as seed treatment for wheat improved seed germination, germination index, vitality index, fresh weight, root/shoot ratio and seedling index (Li et al. 2019). The optimum wheat seedling growth effect was reported at a concentration of NPs (5 μg/mL), 1000 times lower than those used in this present study. Non-loaded γ-polyglutamic acid/chitosan (γ-PGA/CS) nanoparticles showed no effect on the germination of Phaseolus vulgaris when studied as a delivery system with nanoencapsulated gibberellic acid for seed treatment (Pereira et al. 2017). Despite the preponderant beneficial effect of chitosan NPs, a contradictory result was observed for chitosan/tripolyphosphate (CS/TPP) nanoparticles, where NP water solutions were used as germination media for Zea mays, Brassica rapa, and Pisum sativum seeds (Nakasato et al. 2017). CS/TPP NPs hindered germination, with a total inhibition for B. rapa and Z. mays at higher concentrations, significantly reducing the germination rate by 80–90% of P. sativum. In the same study, solid lipid NPs exhibited no germination inhibition within the studied concentration ranges.
There were no observable differences in seed germination between various treatments in the present study, regardless of the nature of the particles used, their size, charge, or the applied concentration. No significant impacts on germination rates were observed when soybean seeds were treated with ZNPs or LNPs. The emergence of most soybean seeds occurred in the first 5 days after sowing with the remaining seeds surfacing within 10 days. Seed viability was not significantly affected by treatments when compared to the non-treated control (Fig. 2A). Earlier, a positive effect was reported for maize seeds treated with tebuconazole microencapsulated in hydroxypropyl cellulose (Yang et al. 2014). These results were attributed to the microencapsulated form of the antifungal agent, although the effects of non-loaded polymeric microcapsules were not assessed in the study.
Root length and dry biomass
Root lengths were statistically similar to the control (Fig. 2B), with a slight decrease in length for ZNP-D at 5 mg/mL (~ 13%), ZNP-T at 10 mg/mL (~ 19%) and LNP at 10 mg/ml (~ 19%). In general, plants treated with PNPs showed an increase in root biomass, albeit nonsignificant, from the control, ZNP-T at 5 and 10 mg/mL (~ 20%), and LNP at 5 mg/mL (~ 25%) (Fig. 2B). The increase in dry root biomass and associated decrease in length may be attributed to a more developed secondary root system, resulting in a shorter primary root but greater average albeit not significantly different biomass than the controls. NPs have been theorized to block entryways for water and nutrient absorption by adhering to the surface of the cell wall, as seen in a previous study focused on plant roots (Buono et al. 2021). This mechanism could be a source of plant stress in soybean plants treated with both lignin and zein nanoparticles (Salinas et al. 2021). As a response, the plants may have adapted by growing a more developed secondary root system to obtain nutrients necessary for survival. Alternatively, as discussed earlier, the biochemical effects of lignin may have positively impacted early plant development, resulting in increased biomass. Similar results were reported for non-loaded CSNPs (Li et al. 2019), increasing fresh weight and the number of adventitious roots of wheat seedlings, assessed after in vitro germination. An increase of fresh weight of maize seedling roots was also observable for microencapsulated tebuconazole, where no data are available for the non-loaded hydroxypropyl cellulose microcapsules contribution to this effect (Yang et al. 2014). P. vulgaris seeds treated with non-loaded γ-PGA/CS NPs showed no effect on plant growth compared to control, although the same particles loaded with gibberellic acid improved root formation (Pereira et al. 2017).
Stem length and dry biomass
Compared to controls, stem lengths were similar (Fig. 2C), with a slight decrease for LNP-treated seeds at 5 mg/mL (~ 23%, P = 0.04, due to some marginal values, although this decrease is not reflected in the biomass), a non-significant decrease for LNP-treated seeds at 10 mg/mL (~ 13%), and a small increase for ZNP-D at 5 mg/ml (~ 15%, P = 0.04). Stem biomasses were similar to the control (Fig. 2C), except for plants treated with LNPs at 10 mg/mL, where a small decrease was observed (~ 20%). Despite these differences between the control and treatment groups, plant morphology and viability were visually similar, suggesting the PNP treatments did not hinder plant development. Other studies involving PNPs mostly reported no negative outcome of treatments (Li et al. 2019; Pereira et al. 2017), with the exception of CS/TPP nanoparticles tested on Z. mays, B. rapa, and P. sativum seeds (Nakasato et al. 2017). Not only was germination completely halted under certain conditions, but plant development was also significantly hindered. In contrast, seed treatment with non-loaded CSNPs promoted wheat seedling length after in vitro germination at lower concentrations and had no negative effect at higher concentrations (Li et al. 2019). In addition to improved seed germination indexes, an increase in fresh weight was also observable for CSNPs treated seedlings. Another study involving chitosan-based, γ-PGA/CS nanoparticles, found no effect of non-loaded particles on the growth parameters of P. vulgaris (Pereira et al. 2017). Meanwhile, microencapsulated tebuconazole is known to stimulate the growth of maize seedling shoots. However, it is not certain if the effect is solely due to the novel formulation of tebuconazole or it also involves the delivery system, the hydroxypropyl cellulose microcapsules (Yang et al. 2014).
Chlorophyll content
According to SPAD measurements, the chlorophyll content of treated soybeans was similar to those of non-treated soybeans, with an average value of 31.6. Soybeans treated with LNPs showed a decrease in chlorophyll content of ~ 15% compared to the controls (Fig. 2D), which was statistically significant at the concentration of 5 mg/mL (P < 0.05). The cause behind this phenomenon can be attributed to the blockage of nutrient uptake by LNP adsorption on the soybean surface. It is possible that this mechanism could have limited the uptake of essential nutrients necessary for chlorophyll synthesis, such as magnesium and iron, resulting in a lower chlorophyl content in the leaves (Buono et al. 2021). Treatment of soybean seeds with high concentrations of PNPs (5–10 mg/mL) overall had minimal effect on seed and plant health in the present experiment. Other studies (Li et al. 2019; Nakasato et al. 2017; Pereira et al. 2017) reported changes in plant health, even though the tested concentration ranges were much lower; however, these studies focused on particles that were different from ZNPs and LNPs in their composition and physical chemical characteristics. For example, CSNP nanoparticles at low concentrations (1 μg/mL, 10 μg/mL) did not affect the chlorophyll content of the treated wheat seedlings but higher particle concentrations (50 μg/mL, 100 μg/mL) lead to a decrease in chlorophyll content (Li et al. 2019). Tebuconazole microcapsule formulation treatments significantly increased the carotenoid and chlorophyll content in maize seedlings. As no data are available on non-loaded microcapsules, changes are attributed to the active ingredient (Yang et al. 2014).
This study indicated that ZNP and LNP seed treatments had little to no effect on seed viability and plant growth of soybeans treated with high concentrations of PNPs. Some marginal differences were observed in plant growth parameters between control plants and treated plants, but plant viability was not significantly affected overall. Although statistically significant differences were recorded at some NP concentrations, no correlation or specific dose dependence could be detected.
PNP tracking
Fluorescence microscopy was performed to track the association of PNPs with soybean seeds after treatment and after 1 day of germination. A 1-h release study was performed to ensure that the observed fluorescence came from the PNPs rather than the free fluorophore (results not shown). This study indicated that a significant amount of FITC was not released from LNP during the treatment time of the experiment (5 min) and could, therefore, be utilized for PNP tracking. Due to the covalent attachment of FITC to zein, it was assumed that a significant amount of the fluorophore would not be released from ZNP within 5 min. Imaging of non-germinated seeds showed that ZNPs adhered to the surface of the seed coat (Fig. 3B′, C′). Both ZNP-D and ZNP-T particles are positively charged, granting them an affinity for the negatively charged surface of cell walls (Spielman-Sun et al. 2017). Thus, the majority of NPs primarily adhere to the first surface they contact. Ruptures appear in the seed coat as it imbibes water during treatment, enabling the ZNPs to enter under the seed coat. Due to the ZNPs’ high affinity to the surface of the cell wall, there is no detectable nanoparticle penetration inside the cotyledons, only a slight adherence on the first cell layer of the endosperm. Lignin nanoparticles, however, are negatively charged and do not adhere in the same manner to the exterior of the seed coat. The smaller, negatively charged LNPs’ coat uniformly the seed coat’s exterior and interior layer and the endosperm’s first layer (Fig. 3D′), as the NP enters between the seed coat and cotyledons during treatment.
Cross-sections of non-germinated soybean seeds visualized by brightfield microscopy (first row): A control, B FZNP-D treated, C FZNP-T treated, D FLNP treated; and by fluorescence microscopy, using a filter for FITC showing fluorescently tagged PNPs association on soybean seeds (second row): A′ control, B′ FZNP-D treated, C′ FZNP-T, D′ FLNP treated, where: FZNP-D fluorescently tagged ZNPs synthesized with DMAB, FZNP-T fluorescently tagged ZNPs synthesized with Tween 80, FLNP fluorescently tagged lignin-PLGA nanoparticles
During the first 24 h of germination, soybean seeds undergo morphological and chemical transformations (Mostafa et al. 1987). Seeds increase in size as they imbibe water from the environment and specific biochemical processes commence. Seed coats straighten as they become hydrated and mold on the swollen cotyledons; they may present fissures where the radicle starts emerging or when the cotyledons exceed them in size. Still, PNPs did not translocate during these processes from the seed coat to the interior of the cotyledons, as they remained adhered to the seed coat (Fig. 4B′–D′). Similar studies report the presence of FITC-labeled chitosan NPs (CSNPs) on the exterior of wheat seeds after incubation for several hours (Li et al. 2019). No PNPs were detected inside the seed, even after the 60-h incubation period.
Cross-sections of germinated soybean seeds, after 1 day, in brightfield microscopy (first row): A control, B FZNP-D treated, C FZNP-T treated, D FLNP treated; and in fluorescence microscopy, using a filter for FITC (second row) showing fluorescently tagged PNPs association on soybean seeds: A′ control, B′ FZNP-D treated, C′ FZNP-T, D′ FLNP treated, where: FZNP-D fluorescently tagged ZNPs synthesized with DMAB, FZNP-T fluorescently tagged ZNPs synthesized with Tween 80, FLNP fluorescently tagged lignin-PLGA nanoparticles
Assessment of antifungal properties
To assess antifungal effects of fungicide-loaded PNPs, soybean seeds were treated with azoxystrobin-loaded PNPs at a at a 0.5 mg/mL azoxystrobin concentration. Their effectiveness was compared to azoxystrobin dissolved in water and the commercial formulation. Plant emergence was not influenced by any of the treatments (data not shown) in non-inoculated and inoculated treatments, although all inoculated treatments displayed visible lesions caused by R. solani.
Plant health was not affected by non-loaded PNPs, nor azoxystrobin loaded PNPs, as there were no significant differences observed in root and shoot length, and dry biomass of the soybean plants after 14 days from emergence (Fig. 5A, B). However, R. solani still affected the inoculated plants, as a lower biomass was observed than for the non-inoculated plants (Fig. 5B).
A Root and shoot length after 14 days; B dry biomass after 14 days; C lesions on plant stem. Different lowercase letters indicate significant difference at P < 0.05 (n = 16), where NIC control, non-inoculated, soybean seeds treated with water, NIC-A non-inoculated control, soybean seeds treated with azoxystrobin dissolved in water, NIC-CA non-inoculated control, soybean seeds treated with commercial formulation, dynasty, NIC-ZNP-T non-inoculated control, soybean seeds treated with empty ZNPs synthesized with Tween 80, NIC-ZNP-D non-inoculated control, soybean seeds treated with empty ZNPs synthesized with DMAB, NIC-LNP non-inoculated control, soybean seeds treated with empty LNPs, NIC-ZNP-TA non-inoculated control, soybean seeds treated with ZNPs synthesized with Tween 80 and entrapped azoxystrobin, NIC-ZNP-DA non-inoculated control, soybean seeds treated with ZNPs synthesized with DMAB and entrapped azoxystrobin, NIC-LNP-A non-inoculated control, soybean seeds treated with LNPs with entrapped azoxystrobin, IC inoculated control, soybean seeds treated with water, IC-A inoculated control, soybean seeds treated with azoxystrobin dissolved in water, IC-CA inoculated control, soybean seed treated with commercial formulation, Dynasty®, IC-ZNP-T inoculated, soybean seeds treated with empty zein-tween 80 NPs, IC-ZNP-D inoculated, soybean seeds treated with empty zein-DMAB NPs, IC-LNP inoculated, soybean seeds treated with empty lignin-PLGA NPs, IC-ZNP-TA inoculated, soybean seeds treated ZNPs synthesized with Tween 80 and entrapped azoxystrobin, IC-ZNP-DA inoculated, soybean seeds treated with ZNPs synthesized with Tween 80 and entrapped azoxystrobin, IC-LNP-A inoculated, soybean seeds treated with LNPs with entrapped azoxystrobin
The LNPs with entrapped azoxystrobin provided effective protection (close to 100%) compared to all formulations studied at 0.5 mg/mL (Fig. 5C). The commercial formulation provided statistically similar protection, with plants presenting only 20% surface lesions. ZNP-Ds loaded with azoxystrobin showed similar protection to the commercial formulation and LNPs delivered azoxystrobin. Loaded ZNP-Ts were ineffective against R. solani, although they provided slightly better protection than azoxystrobin dissolved in water, where plants presented 50% lesions compared to 80%.
Although azoxystrobin is relatively immobile in soil, it is known that it may translocate to more than 15 cm depth in heavily irrigated soil (Ghosh and Singh 2009), possibly leading to the decrease of its concentration around the treated seeds. Azoxystrobin dissolved in water offered almost no protection for the soybean seeds, whereas PNPs enhanced its antifungal effect through a sustained release at the application site. Pesticide-loaded LNPs offered an improved antifungal protection compared to ZNPs, as the release of the active compound from LNPs is slower and more moderate. LNPs showed an increased stability over time and a sustained release over 50 h of the anticancer drug, MEK1/2 inhibitor GDC-0623 (Byrne et al. 2020) under physiological conditions (phosphate buffer solution, 37 °C), while zein/lutein NPs released 80% of the entrapped lutein in less than an hour in similar conditions (Hu et al. 2012). ZNPs faster release may be accelerated in the soil, due to their proteic nature and susceptibility to the enzymatic digestion of the microbiota. ZNP degradation is also influenced by pH and the surfactant used for synthesis (de Oliveira et al. 2014). As ZNP-T has a shorter persistence time in acidic aqueous media (~ 2000 days, pH 4, 20 °C) than ZNP-D (~ 4000 days, pH 4, 20 °C), it is expected to also degrade faster in soil (pH 5.6) and release azoxystrobin under a much shorter time.
Insect bioassay
When comparing the replicates of the mortality test, it was found that one replicate was significantly different from the others (P < 0.05), and was removed from the analysis. Statistical tests concluded that there were no differences in C. includens neonate mortality between the nine soybean seed treatments (F = 0.83, df = 8, 18, P = 0.56) (Table 2).
This is the first experiment to document the effect of ZNP and LNP treated seeds on an insect pest. Previous research has indicated that positively charged ZNPs significantly impact C. includens mortality at high concentrations (200 PPM) when reared on the treated diet; positively charged ZNPs appear to have a defined LC50 value of 1478 PPM for Anticarsia gemmatalis (Hübner), another lepidopteran insect pest (Bonser et al. 2020). The results from current study, however, suggest that the nanoparticle seed treatments did not affect the mortality of lepidopteran larvae following consumption of leaf tissue of treated seeds.
aMeans followed by the same letter within columns are not significantly different (P > 0.05; Tukey’s HSD)
There is a deficit of research on how nanoparticle seed treatments affect insects, with this study being the first to document the effects of ZNP and LNP treated seed on a lepidopteran soybean pest. Research of antifungal nanoparticle seed treatments appears to suggest strong lethality in insects. For example, in vitro assays of silver nanoparticles inhibited Colletotrichum fungus at concentrations of 100 PPM (Lamsal et al. 2011). However, studies have suggested that the lethality of silver nanoparticles is nearly an order of magnitude lower, being in the range of 3.6–12 PPM in Aedes albopictus (Skuse) (Fouad et al. 2018; Ga’al et al. 2018). As another example, research found that antifungal seed treatments of chitosan-polyacrylic acid nanoparticles on Callosobruchus maculatus (F.), Callosobruchus chinensis L., and Aphis gossypii Glover demonstrated a significant decrease in fecundity by nearly 80% and insect growth by at least 70% (Sahab et al. 2015). The research presented in this paper suggests that ZNP- and LNP-treated seeds do not express mortal effects. In fact, research has indicated that positively charged ZNPs significantly impact C. includens and Anticarsia gemmatalis (Hübner) mortality at high concentrations; 200 PPM and 1478 PPM, respectively (Bonser et al. 2020). The experiment in this paper gives evidence that antifungal seed treatments of ZNP and LNP will not negatively affect insects.
Conclusions
Polymeric nanoparticles were synthesized based on biodegradable polymers, zein and lignin, easily obtained from natural resources. ZNPs and LNPs may have potential use in agricultural applications as seed treatments serving as suitable delivery systems for many active compounds, mainly including fungicides, insecticides, and biological materials. This study indicates that ZNP and LNP seed treatments had little to no effect on seed viability and plant growth of soybeans treated with high concentrations of PNPs. Germination rates were high for all treatments regardless of NP concentration. Some marginal differences were observed in plant growth parameters between control plants and treated plants, but on the whole, plant viability was not significantly affected. Although statistically significant differences were recorded at some NP concentrations, no correlation or specific dose dependence could be detected. Microscopical imaging revealed that PNPs adhered only to the outer and inner surface of the seed coat without penetration in the cotyledons. Thus, ZNP and LNP treatment of seeds was found to not interfere with the germination process and may be suitable for seed treatment delivery systems of active compounds. Azoxystrobin-loaded (technical grade) LNPs offered almost complete antifungal protection (~ 100%) for soybean plants against R. solani when used as a seed treatment, while ZNP-D displayed similar effectiveness as the commercial formulation (~ 80% protection) closely. Although the ZNP-T loaded with azoxystrobin did not offer effective protection, it improved the antifungal effect of free azoxystrobin. Zein and lignin polymer-based NPs open the way towards a safe and natural alternative to improve pesticide use in seed treatment without interfering with plant physiology.
References
Ajayi-Oyetunde OO, Bradley CA (2017) Identification and characterization of Rhizoctonia species associated with soybean seedling disease. Plant Dis 101(4):520–523. https://doi.org/10.1094/PDIS-06-16-0810-RE
Astete CE, De Mel JU, Gupta S, Noh Y, Bleuel M, Schneider GJ, Sabliov CM (2020) Lignin-graft-poly(lactic-co-glycolic) acid biopolymers for polymeric nanoparticle synthesis. ACS Omega 5(17):9892–9902. https://doi.org/10.1021/acsomega.0c00168
Atwood LW, Mortensen DA, Koide RT, Smith RG (2018) Evidence for multi-trophic effects of pesticide seed treatments on non-targeted soil fauna. Soil Biol Biochem 125:144–155. https://doi.org/10.1016/j.soilbio.2018.07.007
Avellan A, Yun J, Morais BP, Clement ET, Rodrigues SM, Lowry GV (2021) Environ Sci Technol 55(20):13417–13431. https://doi.org/10.1021/acs.est.1c00178
Bonser CAR, Chen X, Astete CE, Sabliov CM, Davis JA (2020) Elucidating efficacy of ingested positively charged zein nanoparticles against noctuidae. J Econ Entomol 113(6):2739–2744. https://doi.org/10.1093/jee/toaa199
Buono DD, Luzi F, Puglia D (2021) Lignin nanoparticles: a promising tool to improve maize physiological biochemical and chemical traits. Nanomaterials 11(4):846. https://doi.org/10.3390/nano11040846
Byrne CE, Astete CE, Vaithiyanathan M, Melvin AT, Moradipour M, Rankin SE, Knutson BL, Sabliov CM, Martin EC (2020) Lignin-graft-PLGA drug-delivery system improves efficacy of MEK1/2 inhibitors in triple-negative breast cancer cell line. Nanomedicine 15(10):981–1000. https://doi.org/10.2217/nnm-2020-0010
Campos EVR, De Oliveira JL, Da Silva CMG, Pascoli M, Pasquoto T, Lima R, Abhilash PC, Fernandes Fraceto L (2015) Polymeric and solid lipid nanoparticles for sustained release of carbendazim and tebuconazole in agricultural applications. Sci Rep 5:1–14. https://doi.org/10.1038/srep13809
Cui X, Shizhen M, Liu M, Yuan H (2008) Mechanism of surfactant micelle formation. Langmuir 24(19):10771–10775. https://doi.org/10.1021/la801705y
de Oliveira JL, Campos EVR, Bakshi M, Abhilash PC, Fraceto LF (2014) Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: prospects and promises. Biotechnol Adv 32(8):1550–1561. https://doi.org/10.1016/j.biotechadv.2014.10.010
Fehr WR, Caviness CE (1977) Stages of soybean development (1977). Special report, vol 80. Iowa State University, pp 1–12
Fouad H, Hongjie L, Hosni D, Wei J, Abbas G, Ga’al, H., and Jianchu, M. (2018) Controlling Aedes albopictus and Culex pipiens pallens using silver nanoparticles synthesized from aqueous extract of Cassia fistula fruit pulp and its mode of action. Artif Cells Nanomed Biotechnol 46(3):558–567. https://doi.org/10.1080/21691401.2017.1329739
Ga’al H, Fouad H, Tian J, Hu Y, Abbas G, Mo J (2018) Synthesis, characterization and efficacy of silver nanoparticles against Aedes albopictus larvae and pupae. Pesticide Biochem Physiol 144:49–56. https://doi.org/10.1016/j.pestbp.2017.11.004
Gabriel Paulraj M, Ignacimuthu S, Gandhi MR, Shajahan A, Ganesan P, Packiam SM, Al-Dhabi NA (2017) Comparative studies of tripolyphosphate and glutaraldehyde cross-linked chitosan-botanical pesticide nanoparticles and their agricultural applications. Int J Biol Macromol 104(234081):1813–1819. https://doi.org/10.1016/j.ijbiomac.2017.06.043
Ghosh RK, Singh N (2009) Leaching behaviour of azoxystrobin and metabolites in soil columns. Pest Manag Sci 65(9):1009–1014. https://doi.org/10.1002/ps.1787
Goldsmith PD (2008) Economics of soybean production, marketing, and utilization. Soybeans. https://doi.org/10.1016/B978-1-893997-64-6.50008-1
Grillo R, Mattos BD, Antunes DR, Forini ML, Monikh FA, Rojas OR (2021a) Foliage adhesion and interactions with particulate delivery systems for plant nanobionics and intelligent agriculture. Nano Today 37:101078. https://doi.org/10.1016/j.nantod.2021.101078
Grillo R, Fraceto LF, Amorim M, Scott-Fordmand JJ, Schoonjans R, Chaudhry Q (2021b) Ecotoxicological and regulatory aspects of environmental sustainability of nanopesticides. J Hazard Mater 404:124148. https://doi.org/10.1016/j.jhazmat.2020.124148
Hu D, Lin C, Liu L, Li S, Zhao Y (2012) Preparation, characterization, and in vitro release investigation of lutein/zein nanoparticles via solution enhanced dispersion by supercritical fluids. J Food Eng 109(3):545–552. https://doi.org/10.1016/j.jfoodeng.2011.10.025
Hu P, An J, Faulkner MM, Wu H, Li Z, Tian X, Giraldo JP (2020) Nanoparticle charge and size control foliar delivery efficiency to plant cells and organelles. ACS Nano 14(7):7970–7986. https://doi.org/10.1021/acsnano.9b09178
Kacsó T, Neaga IO, Erincz A, Astete CE, Sabliov CM, Oprean R, Bodoki E (2018) Perspectives in the design of zein-based polymeric delivery systems with programmed wear down for sustainable agricultural applications. Polym Degrad Stab 155:130–135. https://doi.org/10.1016/j.polymdegradstab.2018.07.014
Kumar S, Kumar D, Dilbaghi N (2017) Preparation, characterization, and bio-efficacy evaluation of controlled release carbendazim-loaded polymeric nanoparticles. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-016-7774-y
Lamsal K, Kim SW, Jung JH, Kim YS, Kim KS, Lee YS (2011) Application of silver nanoparticles for the control of Colletotrichum species in vitro and pepper anthracnose disease in field. Mycobiology 39(3):194–199. https://doi.org/10.5941/MYCO.2011.39.3.194
Li R, He J, Xie H, Wang W, Bose SK, Sun Y, Hu J, Yin H (2019) Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.). Int J Biol Macromol 126:91–100. https://doi.org/10.1016/j.ijbiomac.2018.12.118
Lichtenberg SS, Laisney J, Elhaj Baddar Z, Tsyusko OV, Palli SR, Levard C, Masion A, Unrine JM (2020) Comparison of nanomaterials for delivery of double-stranded RNA in Caenorhabditis elegans. J Agric Food Chem 68(30):7926–7934. https://doi.org/10.1021/acs.jafc.0c02840
Mostafa MM, Rahma EH, Rady AH (1987) Chemical and nutritional changes in soybean during germination. Food Chem 23(4):257–275. https://doi.org/10.1016/0308-8146(87)90113-0
Nakasato DY, Pereira AES, Oliveira JL, Oliveira HC, Fraceto LF (2017) Evaluation of the effects of polymeric chitosan/tripolyphosphate and solid lipid nanoparticles on germination of Zea mays, Brassica rapa and Pisum sativum. Ecotoxicol Environ Saf 142:369–374. https://doi.org/10.1016/j.ecoenv.2017.04.033
Nettles R, Watkins J, Ricks K, Boyer M, Licht M, Atwood LW, Peoples M, Smith RG, Mortensen DA, Koide RT (2016) Influence of pesticide seed treatments on rhizosphere fungal and bacterial communities and leaf fungal endophyte communities in maize and soybean. Appl Soil Ecol 102:61–69. https://doi.org/10.1016/j.apsoil.2016.02.008
Nuruzzaman M, Rahman MM, Liu Y, Naidu R (2016) Nanoencapsulation, nano-guard for pesticides: a new window for safe application. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.5b05214
Pascoli M, Lopes-Oliveira PJ, Fraceto LF, Seabra AB, Oliveira HC (2018) State of the art of polymeric nanoparticles as carrier systems with agricultural applications: a minireview. Energy Ecol Environ 3(3):137–148. https://doi.org/10.1007/s40974-018-0090-2
Pereira AES, Sandoval-Herrera IE, Zavala-Betancourt SA, Oliveira HC, Ledezma-Pérez AS, Romero J, Fraceto LF (2017) γ-Polyglutamic acid/chitosan nanoparticles for the plant growth regulator gibberellic acid: characterization and evaluation of biological activity. Carbohydr Polym 157:1862–1873. https://doi.org/10.1016/j.carbpol.2016.11.073
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing
Rao NK, Hanson J, Dulloo ME, Ghosh K, Nowell D, Larinde M (2006) Manual of seed handling in genebanks (handbooks, issue handbooks for genebanks no. 8). Bioversity International
Ristroph KD, Astete CE, Bodoki E, Sabliov CM (2017) Zein nanoparticles uptake by hydroponically grown soybean plants. Environ Sci Technol 51(24):14065–14071. https://doi.org/10.1021/acs.est.7b03923
RStudio Team (2020) RStudio: Integrated Development for R. RStudio. PBC
Sahab AF, Waly AI, Sabbour MM, Nawar LS (2015) Synthesis, antifungal and insecticidal potential of chitosan (CS)-g-poly (acrylic acid) (PAA) nanoparticles against some seed borne fungi and insects of soybean. Int J ChemTech Res 8(2):589–598
Saharan V, Sharma G, Yadav M, Choudhary MK, Sharma SS, Pal A, Raliya R, Biswas P (2015) Synthesis and in vitro antifungal efficacy of Cu-chitosan nanoparticles against pathogenic fungi of tomato. Int J Biol Macromol 75:346–353. https://doi.org/10.1016/j.ijbiomac.2015.01.027
Salinas F, Astete CE, Waldvogel JH, Navarro S, White JC, Elmer W, Tamez C, Davis JA, Sabliov CM (2021) Effects of engineered lignin-graft-PLGA and zein-based nanoparticles on soybean health. NanoImpact 23:100329. https://doi.org/10.1016/J.IMPACT.2021.100329
Sharma S, Muddassir M, Muthusamy S, Vaishnav PK, Singh M, Sharma D, Kanagarajan S, Shanmugam V (2020) A non-classical route of efficient plant uptake verified with fluorescent nanoparticles and root adhesion forces investigated using AFM. Sci Rep 10:19233. https://doi.org/10.1038/s41598-020-75685-3
Smalling KL, Hladik ML, Sanders CJ, Kuivila KM (2018) Leaching and sorption of neonicotinoid insecticides and fungicides from seed coatings. J Environ Sci Health Part B Pestic Food Contam Agric Wastes 53(3):176–183. https://doi.org/10.1080/03601234.2017.1405619
Spielman-Sun E, Lombi E, Donner E, Howard D, Unrine JM, Lowry GV (2017) Impact of surface charge on cerium oxide nanoparticle uptake and translocation by wheat (Triticum aestivum). Environ Sci Technol 51(13):7361–7368. https://doi.org/10.1021/acs.est.7b00813
Takeshita V, De Sousa BT, Preisler AC, Carvalho LB, Pereira ADES, Tornisielo VL, Dalazen G, Oliveira HC, Fraceto F (2021) Foliar absorption and field herbicidal studies of atrazine-loaded polymeric nanoparticles. J Hazard Mater 418:126350. https://doi.org/10.1016/j.jhazmat.2021.126350
Tong Y, Wu Y, Zhao C, Xu Y, Lu J, Xiang S, Zong F, Wu X (2017) Polymeric nanoparticles as a metolachlor carrier: water-based formulation for hydrophobic pesticides and absorption by plants. J Agric Food Chem 65(34):7371–7378. https://doi.org/10.1021/acs.jafc.7b02197
Torrent L, Iglesias M, Marguí E, Hidalgo M, Verdaguer D, Llorens L, Kodre A, Kavčič A, Vogel-Mikuš K (2020) Uptake, translocation and ligand of silver in Lactuca sativa exposed to silver nanoparticles of different size, coatings and concentration. J Hazard Mater 384:121201. https://doi.org/10.1016/j.jhazmat.2019.121201
Ur Rahim H, Qaswar M, Uddin M, Giannini C, Herrera ML, Rea G (2021) Nano-enable materials promoting sustainability and resilience in modern agriculture. Nanomaterials 11(8):2068. https://doi.org/10.3390/nano11082068
Yang D, Wang N, Yan X, Shi J, Zhang M, Wang Z, Yuan H (2014) Microencapsulation of seed-coating tebuconazole and its effects on physiology and biochemistry of maize seedlings. Colloids Surf B 114:241–246. https://doi.org/10.1016/j.colsurfb.2013.10.014
Zhang B, Luo Y, Wang Q (2011) Effect of acid and base treatments on structural, rheological, and antioxidant properties of α-zein. Food Chem 124(1):210–220. https://doi.org/10.1016/j.foodchem.2010.06.019
Zhang Y, Fu L, Li S, Yan J, Sun M, Giraldo JP, Matyjaszewski K, Tilton RD, Lowry G (2021) Star polymer size charge content and hydrophobicity affect their leaf uptake and translocation in plants. Environ Sci Technol 55(15):10758–10768. https://doi.org/10.1021/acs.est.1c01065
Funding
Funding was provided by Fulbright Association, National Institute of Food and Agriculture (Grant nos. 2019-67021-29449, 1008750), National Science Foundation (Grant no. OIA 1632854) and Louisiana Soybean and Grain Research Board.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Statement of human and animal rights
The research did not involve Human Participants or Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kacsó, T., Hanna, E.A., Salinas, F. et al. Zein and lignin-based nanoparticles as soybean seed treatment: translocation and impact on seed and plant health. Appl Nanosci 12, 1557–1569 (2022). https://doi.org/10.1007/s13204-021-02307-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-021-02307-3