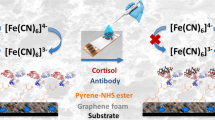
Abstract
Stress nowadays is one of the major causes of various pathologies in humans. Monitoring of stress associated biomarker levels in biological fluids on demand can help in effective management of stress. Since cortisol has been recognised as a potential biomarker for stress, its detection can help in an appropriate therapeutic intervention or stress management. Various types of biosensors such as immunosensors, aptasensors, molecularly imprinted polymers and enzyme-linked based biosensors have been reported till now for the cortisol sensing. Out of all, aptasensors have shown various advantages over other sensors such as specificity, high room temperature stability, reproducibility, etc. Among various reported aptasensors, full-length aptamer (> 60-mer) has been used. However, only a portion of aptamer interact with its target, therefore, establishing its structure–activity relationship is vital for a biosensor to achieve an optimized performance. In the current work, we have rationally truncated already existing 61-mer aptamer sequence into its smaller variant with only 14 bases. Parent and truncated aptamers were immobilized on the graphene quantum dots modified electrodes for electrochemical biosensing of cortisol. Detection limits and cross-reactivities of both the aptamers were compared and it was found that truncated aptamer showed better results in terms of specificity. Though the limit of detection is same for both parent and truncated aptamers, i.e. 0.1 pg/ml but cross-reactivity from various structural analogues in sensing is reduced drastically in case of truncated variant. In brief, we are reporting a label-free electrochemical aptasensor utilizing the truncated aptamer (14-mer) that has remarkable limit of detection (0.1 pg/ml), high level of selectivity and reproducibility for the direct analysis of the cortisol.








Similar content being viewed by others
References
Aberle J, Zur Wiesch CS, Flitsch J, Veigel J, Schön G, Jung R, Reining F, Lautenbach A, Rotermund R, Riedel N (2018) Specificity of late-night salivary cortisol measured by automated electrochemiluminescence immunoassay for Cushing’s disease in an obese population. J Endocrinol Investig 41(11):1325–1331
Abraham GE, Buster JE, Teller RC (1972) Radioimmunoassay of plasma cortisol. Anal Lett 5(11):757–765
Baluta S, Lesiak A, Cabaj J (2018) Graphene quantum dots-based electrochemical biosensor for catecholamine neurotransmitters detection. Electroanalysis 30(8):1781–1790
Bhatnagar D, Kaur I, Kumar A (2017) Ultrasensitive cardiac troponin I antibody based nanohybrid sensor for rapid detection of human heart attack. Int J Biol Macromol 95:505–510
Dalirirad S, Han D, Steckl AJ (2020) Aptamer-based lateral flow biosensor for rapid detection of salivary cortisol. ACS Omega 5(51):32890–32898
Das R, Dhiman A, Mishra SK, Haldar S, Sharma N, Bansal A, Ahmad Y, Kumar A, Tyagi JS, Sharma TK (2019) Structural switching electrochemical DNA aptasensor for the rapid diagnosis of tuberculous meningitis. Int J Nanomed 14:2103
De Kloet ER, Joëls M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6(6):463–475
Dhiman A, Anand A, Malhotra A, Khan E, Santra V, Kumar A, Sharma TK (2018) Rational truncation of aptamer for cross-species application to detect krait envenomation. Sci Rep 8(1):1–8
Dhull N, Kaur G, Gupta V, Tomar M (2019) Highly sensitive and non-invasive electrochemical immunosensor for salivary cortisol detection. Sens Actuators B Chem 293:281–288
Ding Y, Cheng H, Zhou C, Fan Y, Zhu J, Shao H, Qu L (2012) Functional microspheres of graphene quantum dots. Nanotechnology 23(25):255605
Dobson H, Smith R (2000) What is stress, and how does it affect reproduction? Anim Reprod Sci 60:743–752
Dong Y, Shao J, Chen C, Li H, Wang R, Chi Y, Lin X, Chen G (2012) Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 50(12):4738–4743
Eda G, Lin YY, Mattevi C, Yamaguchi H, Chen HA, Chen IS, Chen CW, Chhowalla M (2010) Blue photoluminescence from chemically derived graphene oxide. Adv Mater 22(4):505–509
Elio F, Antonelli G, Benetazzo A, Prearo M, Gatti R (2009) Human saliva cortisone and cortisol simultaneous analysis using reverse phase HPLC technique. Clin Chim Acta 405(1–2):60–65
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346(6287):818–822
Fernandez RE, Umasankar Y, Manickam P, Nickel JC, Iwasaki LR, Kawamoto BK, Todoki KC, Scott JM, Bhansali S (2017) Disposable aptamer-sensor aided by magnetic nanoparticle enrichment for detection of salivary cortisol variations in obstructive sleep apnea patients. Sci Rep 7(1):1–9
Ferrari A, Robertson J (2001) Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys Rev B 64(7):075414
Gn CJ, Eissa S, Ng A, Alhadrami H, Zourob M, Siaj M (2015) Aptamer-based label-free impedimetric biosensor for detection of progesterone. Anal Chem 87(2):1075–1082
Gu J, Zhang X, Pang A, Yang J (2016) Facile synthesis and photoluminescence characteristics of blue-emitting nitrogen-doped graphene quantum dots. Nanotechnology 27(16):165704
Hellhammer DH, Wüst S, Kudielka BM (2009) Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 34(2):163–171
Jayasena SD (1999) Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin Chem 45(9):1628–1650
Ke CC, Yang YC, Tseng WL (2016) Synthesis of blue-, green-, yellow-, and red-emitting graphene-quantum-dot-based nanomaterials with excitation-independent emission. Part Part Syst Charact 33(3):132–139
Kypr J, Kejnovská I, Renčiuk D, Vorlíčková M (2009) Circular dichroism and conformational polymorphism of DNA. Nucleic Acids Res 37(6):1713–1725
Lewis J, Elder P (1985) An enzyme-linked immunosorbent assay (ELISA) for plasma cortisol. J Steroid Biochem 22(5):673–676
Liu X, Hsu SP, Liu W-C, Wang Y-M, Liu X, Lo C-S, Lin Y-C, Nabilla SC, Li Z, Hong Y (2019) Salivary electrochemical cortisol biosensor based on tin disulfide nanoflakes. Nanoscale Res Lett 14(1):1–9
Liu Y, Wu B, Tanyi EK, Yeasmin S, Cheng L-J (2020) Label-free sensitive detection of steroid hormone cortisol based on target-induced fluorescence quenching of quantum dots. Langmuir 36(27):7781–7788
Macdonald J, Houghton P, Xiang D, Duan W, Shigdar S (2016) Truncation and mutation of a transferrin receptor aptamer enhances binding affinity. Nucleic Acid Ther 26(6):348–354
Moghadam FM, Bigdeli M, Tamayol A, Shin SR (2021) TISS nanobiosensor for salivary cortisol measurement by aptamer Ag nanocluster SAIE supraparticle structure. Sens Actuators B Chem 344:130160
Pali M, Jagannath B, Lin K-C, Upasham S, Sankhalab D, Upashama S, Muthukumar S, Prasad S (2021) CATCH (Cortisol Apta WATCH):‘bio-mimic alarm’ to track anxiety, stress immunity in human sweat. Electrochim Acta 390:138834
Pan D, Zhang J, Li Z, Wu M (2010) Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv Mater 22(6):734–738
Sanghavi BJ, Moore JA, Chávez JL, Hagen JA, Kelley-Loughnane N, Chou C-F, Swami NS (2016) Aptamer-functionalized nanoparticles for surface immobilization-free electrochemical detection of cortisol in a microfluidic device. Biosens Bioelectron 78:244–252
Sharma TK, Bruno JG, Dhiman A (2017) ABCs of DNA aptamer and related assay development. Biotechnol Adv 35(2):275–301
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249(4968):505–510
Tuteja SK, Ormsby C, Neethirajan S (2018) Noninvasive label-free detection of cortisol and lactate using graphene embedded screen-printed electrode. Nano-Micro Lett 10(3):41
Whitworth JA, Williamson PM, Mangos G, Kelly JJ (2005) Cardiovascular consequences of cortisol excess. Vasc Health Risk Manag 1(4):291
Zhang J, Gopinath SC (2020) Quantification of cortisol for the medical diagnosis of multiple pregnancy-related diseases. 3 Biotech 10(2):1–10
Zhang Y, Wei Q (2016) The role of nanomaterials in electroanalytical biosensors: a mini review. J Electroanal Chem 781:401–409
Zhang P, Zhao X, Ji Y, Ouyang Z, Wen X, Li J, Su Z, Wei G (2015) Electrospinning graphene quantum dots into a nanofibrous membrane for dual-purpose fluorescent and electrochemical biosensors. J Mater Chem B 3(12):2487–2496
Zhao J, Chen G, Zhu L, Li G (2011) Graphene quantum dots-based platform for the fabrication of electrochemical biosensors. Electrochem Commun 13(1):31–33
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31(13):3406–3415
Acknowledgements
The author VS acknowledges the Department of Science and Technology (DST), Government of India, for INSPIRE fellowship (IF170339). Authors acknowledge Mr. Digvijay Singh Naruka for performing circular dichroism and isothermal titration colorimetry experiments at CSIR-IMTECH, Chandigarh. TKS acknowledge Department of Biotechnology (DBT) for funding support through Translational Research Program (BT/PR30159/MED/15/188/2018).
Author information
Authors and Affiliations
Contributions
VS: conceptualization, methodology, experimentation, data curation and writing—original draft preparation. TKS: conceptualization, supervision, data interpretation, writing—manuscript reviewing and editing. IK: supervision, data analysis, writing—manuscript reviewing and editing.
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, V., Sharma, T.K. & Kaur, I. Electrochemical detection of cortisol on graphene quantum dots modified electrodes using a rationally truncated high affinity aptamer. Appl Nanosci 11, 2577–2588 (2021). https://doi.org/10.1007/s13204-021-02086-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-021-02086-x