Abstract
Zinc oxide nanoflowers (ZnONFs) were prepared by employing the pod extract of Peltophorum pterocarpum as a green resource and characterized by various methods. UV–vis spectrum displayed a peak at 361 nm which confirmed the formation of ZnO nanoparticles. The optical band gap was calculated as 3.43 eV. FE-SEM images exposed the flower-like morphology and EDX portrayed strong signals for Zn and O. XRD studies substantiated signature peaks for the wurtzite phase of ZnONFs and the lattice parameters matched well with the literature. Mesoporous nature was confirmed by BET analysis which yielded a high specific surface area of 19.61 m2/g. FTIR bands at 420.48 and 462.92 cm−1affirmed the Zn and O bonding vibrations. The photocatalytic potential of the ZnONFs was successfully examined for the removal of methylene blue dye under natural solar light. The experimental data were fitted to Langmuir–Hinshelwood’s first-order equation and the kinetic constant was calculated as 0.0114 min–1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is emerging as a forerunner in many fields in recent times. Due to the unique physicochemical properties as compared to their bulk materials, the nanomaterials are extensively explored in various domains such as food, textile, cosmetics, electronics, medical sciences, energy, construction, and environmental remediation (Varadavenkatesan et al. 2020a, b; Pandey et al. 2020a). Zinc oxide nanoparticles (ZONPs) are one such valuable metal oxide nanoparticles which have reaped attention at present due to its unique characteristics like wide bandgap, catalytic efficiency, and nontoxic nature. ZONPs are extensively used as adsorbents, photocatalysts, anti-microbial agents, drug-delivery agents, self-cleansing agents, and semiconductors (Mirzaei and Darroudi 2017).
Several conventional approaches such as chemical, physical, and electrochemical methods are used in the preparation of ZONPs. However, these popular strategies are not cost-effective and face difficulties during purification due to the formation of hazardous by-products. Moreover, the researchers also faced additional important problems such as the achievement of monodispersity and control over the morphology during the preparation of nanoparticles. To eliminate these problems, green resources such as plant extracts and microbial extracts were explored and proven as outstanding substitutes for the preparation of ZONPs (Vinayagam et al. 2020a). The synthesis processes involving these green resources are very rapid, cheap, and easily scalable. The various biomolecules present in these extracts reduce and stabilize the nanoparticles (Groiss et al. 2017). Few examples of green resources used for the preparation of ZONPs include Linum usitatissimum (Alkasir et al. 2020), Moringa oleifera (Letsholathebe et al. 2020), Salvadora persica (Alharthi et al. 2020), Ilex paraguariensis (Bandeira et al. 2020), Sambucus spp. (Alamdari et al. 2020) Abelmoschus esculentus (Mirgane et al. 2020), Peltophorum pterocarpum (Pai et al. 2019a), Cyanometra ramiflora (Varadavenkatesan et al. 2019) and Calliandra haematocephala (Vinayagam et al. 2020b).
Wastewaters loaded with industrial dye effluents are a major environmental concern (Pandey 2017). An example of a cationic dye in methylene blue that has far-reaching applications in diverse areas such as textile, paper and pharmaceutical sectors. It is imperative that the dye is sufficiently removed prior to the waters entering the aquatic bodies as the dye poses critical ecological and health concerns (Pandey et al. 2020b). The usual methods employed for dye elimination (e.g. adsorption) suffer from a lot of disadvantages including cost and pace of the dye transfer. Our research group has already explored the usage of the Peltophorum pterocarpum pod extract to prepare silver (Raja et al. 2015a) and iron oxide nanoparticles (Dash et al. 2019). As demonstrated in these reports, the biomolecules of the extract were responsible for the reduction and stabilization of the nanoparticles.
Therefore, in the current study, we focused on the (1) fabrication of ZONPs by utilizing the P.pterocarpum pod extract, (2) characterization, and (3) photocatalytic ability to decolorize a model pollutant—methylene blue (MB) dye in the presence of solar irradiation.
Materials and methods
Materials
Analytical grade zinc acetate dihydrate, NaOH, and MB dye were purchased from Merck, India, and used without any further purification. Distilled water was used for all the experiments. Fresh pods of P. pterocarpum tree were collected from Manipal, India.
Peltophorum pterocarpum pod extract preparation
P. pterocarpum pod extract (PPPE) was prepared by referring to our earlier reports (Dash et al. 2019; Raja et al. 2015b). Briefly, the pods of P. pterocarpum were first washed with distilled water to remove the surface dirt and then air-dried. The cleaned pods were boiled with water (1:10) to yield a light-yellow extract indicating the release of the polyphenols from the plant material. After cooling and filtering, the extract was collected and preserved in the refrigerator for future use.
Production of zinc oxide nanoflowers (PP-ZnONFs)
Nanoparticles were synthesized by mixing 50 mL of zinc acetate dihydrate (50 mM) and 10 mL of PPPE and subsequent addition of 10 mL NaOH (2 M) to form colloidal suspension on heating. Settling of suspension was observed in a course of time resulting in precipitated ZnONFs. After multiple water-washes, the particles were centrifuged and oven-dried (at 95 °C for 7 h) to obtain pure PP-ZnONFs. The dried powder was pulverized using a mortar and pestle and stored in a plastic vial.
Photocatalytic activity of PP-ZnONFs to degrade MB dye
The photocatalytic potential of the PP-ZnONFs was studied by the degradation of MB dye under natural solar light. The photocatalytic experimentations were performed in a glass beaker. Initially, 40 mg of PP-ZnONFs were mixed with 100 mL of MB dye (25 ppm) and stirred uniformly for 1 h in dark to finish adsorption–desorption process. Afterward, the contents were placed under solar irradiation on a sunny day between 10:30 AM and 3:00 PM with constant stirring. At fixed time intervals, samples were collected and the MB dye concentrations were analyzed by recording the absorption intensity (λmax = 663 nm) using UV–vis spectroscopy.
Characterization
The synthesized PP-ZnONFs were characterized using many sophisticated methods. The synthesis of PP-ZnONFs was monitored by the absorption spectrum of colloidal suspension using a UV–visible spectrophotometer (SHIMADZU—UV1700). Field Emission Scanning Electron Microscope (FE-SEM) (Carl ZEISS, Sigma, Oxford Instruments) was used to examine the individual elements and morphology of the PP-ZnONFs. Pore volume and specific surface area (SSA) were determined using the Brunauer–Emmett and Teller (BET) apparatus (Smart Instruments, Mumbai). X-ray diffraction (Rigaku Miniflex 600) spectrum was used to analyze the crystalline type. Fourier Transform Infrared Spectroscopy (FTIR-SHIMADZU-8400S) was used to investigate the specific functional moieties of the PP-ZnONFs.
Results and discussions
Visual examination and UV–vis analysis
The confirmatory spot check for the development of PP–ZnONFs was the color change of the reaction mixture when the pale yellow PPPE (Fig. 1A) was mixed with the colorless zinc acetate solution (Fig. 1B). This leads to the formation of a colloidal suspension (Fig. 1C) upon heating. The subsequent addition of NaOH leads to a white precipitate (Fig. 1D) in due course. These observations were in concordance with our earlier reports for the fabrication of ZnONPs using various green resources (Varadavenkatesan et al. 2019; Vinayagam et al. 2020b; Pai et al. 2019).
As mentioned earlier, the PPPE contains various types of biomolecules that combine with the metallic salt, zinc acetate to form an intermediate complex through hydrogen bonding (Suresh et al. 2018). Under the basic environment, this forms metal hydroxide which yields zinc oxide nanoparticles while heating. The reduction of Zn was possible and spontaneous because of the fact that the standard reduction potential of biomolecules of plant materials is between 0.3 and 0.8 V whereas for Zn it is just—0.76 V (Haynes 2014). Apart from the reduction, the biomolecules help in the stabilization of nanoparticles by self-assembling and lead to the formation of flower-like nanostructures. A similar mechanism was described for the ZnONFs synthesized using Bacillus licheniformis extract and zinc acetate precursor (Tripathi et al. 2014).
Following the visual observation, the PP–ZnONFs development was confirmed by making a PP-ZnONFs dispersion in water and conducting UV–vis spectral examination of the same. As evinced from Fig. 2, a distinctive absorption peak was observed at 361 nm due to the surface plasmon resonance (SPR) of zinc oxide nanoparticles (Varadavenkatesan et al. 2019). The absence of other peaks substantiated the purity of the ZnONFs. The surface plasmon resonance (SPR) of the ZnONPs alters the particle morphology and dielectric constant of the response channel (Chakraborty et al. 2020). The optical bandgap of PP–ZnONFs was calculated using the Planck-Einstein relationship and found out as 3.43 eV. The value obtained was concordant with the previously reported literature. For example, bandgap values of 3.4 eV and 3.44 eV were obtained in studies using Jatropha gossypifolia leaf extract and Cyanometra ramiflora leaf extracts (Varadavenkatesan et al. 2019; Krishnan et al. 2020).
SEM and EDX studies
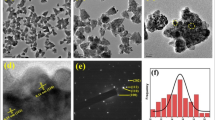
The FESEM image of PP-ZnONFs with the magnification of 20 kX and 80 kX are shown in Fig. 3A and B. Aggregated flower-shaped ZnO-NFs were observed. Clusters of particles were observed due to crystal growth. The formation of aggregations was possibly owing to the divergence, electrostatic attraction, and high surface energy PP-ZnONFs (Darvishi et al. 2019). The presence of organic compounds in the extract may also cause agglomeration (Vinayagam et al. 2020b). The average thickness of the nanoflower petals was calculated as 59 nm using ImageJ software. The observation of the nanoflower-shaped zinc oxide nanoparticles is common for the green synthesis using the extracts of P. pterocarpum (Pai et al. 2019), E. sanguinea (Ekennia et al. 2020) and W. coagulans (Hasan et al. 2021).
The EDX spectra (Fig. 3C) showed the presence of zinc, oxygen, and carbon atoms. To be specific, Zn indicated strong signals at 8.6 keV and 1 keV. Similarly, O exhibited a strong signal at 0.5 keV. Therefore, the existence of these signals corroborated the establishment of ZnO nanoparticles. The C signal might be because of the participation of phytochemicals during the synthesis and capping of ZnONFs (Pai et al. 2019).
The higher weight percentage of Zn (66.59%) and O (24.29%) witnessed the presence of ZnO nanoparticles. Also, the atomic compositions (%) of the individual elements in the sample were observed as, Zn: 30.91, O: 46.06, and C: 23.03. Similar kinds of atomic compositions (%), Zn: 36, O: 44.85 and C: 15.68 were reported for the synthesis of ZnONPs by utilizing Justicia spicigera leaf extract (Soto-Robles et al. 2021). The purity of the ZnO nanoparticles was ascertained by the non-appearance of other peaks.
XRD studies
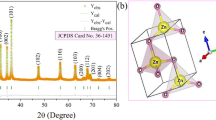
The XRD image of the sample with the Miller indices of peaks is depicted in Fig. 4. The diffraction signals at the 2θ values of 31.31°, 34.06°, 35.84°, 47.20°, 56.23°, 62.48°, 67.62°,68.74° and 76.74° corresponded to (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (1 1 2), (2 0 1) and (2 0 2) Miller indices which were precisely indexed to the pure hexagonal wurtzite phase of zinc oxide nanoparticles (JCPDS Card No.: 36–1451) (Ekennia et al. 2020). The lattice parameters ‘a’ and ‘c’ were computed as 3.269 Å and 5.2092 Å, respectively, which matched with the literature (Pudukudy and Yaakob 2015). The ratio of the lattice parameters “c/a” was equal to 1.594 which matched well with the ZnONPs synthesized using the flower extract of Aspalathus linearis (Diallo et al. 2015).
The sharp and distinct diffraction signals indicated the high crystalline nature of the nanoparticles. No other signals were observed. The full width at half maximum (FWHM), ‘β’ of these peaks yielded a mean crystallite size of 17.79 nm (Table 1) calculated by the Debye–Scherrer equation which matched well with previous studies (Alamdari et al. 2020; Vinayagam et al. 2020b).
BET studies
It is well-known that the flower-like ZnONPs have more SSA as compared to other morphological forms (Luo et al. 2014). The SSA and pore volume were analyzed by BET method. As expected, the PP-ZnONFs showed a high SSA of 19.61 m2/g which was almost 6 times higher than that of commercial ZnO nanoparticles (3.23 m2/g) (Chakrabarti and Dutta 2004). It is apparent from Table 2 that the SSA obtained in the present work is much higher than the other green synthesized ZnO nanoparticles. Besides, the pore volume was 0.0826 cm3/g which confirmed the mesoporous structure (16.85 nm) of pores. Therefore, the high surface area and mesoporous structure of PP-ZnONFs make them vital representatives for many applications.
FTIR studies
The composition of the PP-ZnONFs, with respect to the functional moieties, was deduced from the FTIR spectrum (Fig. 5). The broadband in the range of 3400–3600 cm−1 was mainly due to the stretching vibration of the phenolic hydroxyl group (Darvishi et al. 2019; Soto-Robles et al. 2021; Lu et al. 2019). The band at 2359 cm−1 was due to the C–H stretching vibrations of aromatic aldehyde (Akbarian et al. 2020). A weak band at 1541 cm−1 corresponded to C=C stretching bonds of aromatic compounds in the extract (Vinayagam et al. 2020b; Hu et al. 2019). The band at 1175 cm−1 belonged to C-O stretching (Darvishi et al. 2019; Chen et al. 2019).
The bands at 637, 718, and 781 cm−1 in the fingerprint region corresponded to aromatic C-H groups in the extract (Darvishi et al. 2019). The bands at 421 and 463 cm−1 may be attributed to characteristic Zn and O bonding vibrations (Suresh et al. 2018; Li et al. 2019; Gopalakrishnan et al. 2019). Therefore, the signals for various functional moieties ascertained the contribution of the phytochemicals present in the PPPE for the formation of ZnONFs.
Photocatalytic activity of PP-ZnONFs to degrade MB dye
The UV–vis spectral images of photodegradation of MB dye by PP-ZnONFs were shown in Fig. 6A along with the discoloration of MB dye as a function of time. It can be witnessed from the images that the MB dye solution became colorless after 180 min of solar irradiation. The decrement in the absorbance of MB dye at λmax = 663 nm substantiated the degradation process. From Fig. 6B, it can be inferred that 88.39% of the MB dye was photodegraded in 180 min solar irradiation.
It can be inferred from Table 3 that the degradation efficiency of MB dye by the PP-ZnONFs is much higher than the recent reports on the green synthesized ZnO nanoparticles. This enhanced photocatalytic activity of the PP-ZnONFs may be due to the small particle size, mesoporosity, and high specific surface area.
The kinetic data of the photodegradation was fitted to the Langmuir–Hinshelwood first-order equation as given by,
where “t” is the solar irradiation time in min, A0 and Af are the initial and final concentration of MB dye and kphd is the degradation constant. Figure 6C shows the relationship between \(\ln \left[ {\frac{{A_{{\text{f}}} }}{{A_{0} }}} \right]\;\) and t with linear relationship and the value of kphd was determined as 0.0114 min–1.
The incident sunlight has more energy than the bandgap energy of PP-ZnONFs. Therefore, as depicted in Fig. 7 the photocatalytic mechanism involves the creation of electrons (e–) in the conduction band (CB) and holes (h+) in the valance band (VB) when sunlight irradiates on the surface of PP-ZnONFs. These generated e– converts dissolved O2 to form superoxide free radicals (O2–) and h+ produce ·OH free radicals. These intermediates are highly active which act as oxidizing and reducing agents and thus are responsible for the photodegradation of MB dye as explained by many researchers (Tabrizi Hafez Moghaddas et al. 2019; Ganesh et al. 2019; Pandimurugan and Thambidurai 2016).
Conclusion
Green synthesized Zinc oxide nanoflowers (ZnONFs) have been synthesized by utilizing the pod extract of Peltophorum pterocarpum for the first time. The formation of ZnO nanoparticles was confirmed by the characteristic signals shown in UV–vis spectrum, EDX image, XRD pattern, and FTIR spectrum. FE-SEM image confirmed the flower-like nanostructures with few aggregations and BET analysis ascertained the mesoporosity with a relatively higher specific surface area than commercial ZnO nanoparticles. 88.39% of MB dye was photodegraded under sunlight within 180 min in the presence of ZnONFs. Therefore, the green-synthesized ZnONFs obtained in the present study can play a significant role in the removal of harmful dyes present in industrial wastewater.
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
Akbarian M, Mahjoub S, Mohammad S, Zabihi E (2020) Biointerfaces Green synthesis, formulation and biological evaluation of a novel ZnO nanocarrier loaded with paclitaxel as drug delivery system on MCF-7 cell line. Colloids Surf B Biointerfaces 186:110686. https://doi.org/10.1016/j.colsurfb.2019.110686
Alamdari S, Ghamsari MS, Lee C, Han W, Park HH, Tafreshi MJ, Afarideh H, Ara MHM (2020) Preparation and characterization of zinc oxide nanoparticles using leaf extract of sambucus ebulus. Appl Sci 10:1–19. https://doi.org/10.3390/app10103620
Alharthi FA, Alghamdi AA, Alothman AA, Almarhoon ZM, Alsulaiman MF, Al-Zaqri N (2020) Green synthesis of zno nanostructures using salvadora persica leaf extract: applications for photocatalytic degradation of methylene blue dye. Curr Comput-Aided Drug Des. https://doi.org/10.3390/cryst10060441
Alkasir M, Samadi N, Sabouri Z, Mardani Z, Khatami M, Darroudi M (2020) Evaluation cytotoxicity effects of biosynthesized zinc oxide nanoparticles using aqueous Linum Usitatissimum extract and investigation of their photocatalytic activityackn. Inorg Chem Commun 119:108066. https://doi.org/10.1016/j.inoche.2020.108066
Bandeira M, Possan AL, Pavin SS, Raota CS, Vebber MC, Giovanela M, Roesch-Ely M, Devine DM, Crespo JS (2020) Mechanism of formation, characterization and cytotoxicity of green synthesized zinc oxide nanoparticles obtained from Ilex paraguariensis leaves extract. Nano-Struct Nano-Obj 24:100532. https://doi.org/10.1016/j.nanoso.2020.100532
Chakrabarti S, Dutta BK (2004) Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J Hazard Mater 112:269–278
Chakraborty S, Farida JJ, Simon R, Kasthuri S, Mary NL (2020) Averrhoe carrambola fruit extract assisted green synthesis of zno nanoparticles for the photodegradation of congo red dye. Surf Interfaces 19:100488. https://doi.org/10.1016/j.surfin.2020.100488
Chen L, Batjikh I, Hurh J, Han Y, Huo Y, Ali H, Li JF, Rupa EJ, Ahn JC, Mathiyalagan R, Yang DC (2019) Green synthesis of zinc oxide nanoparticles from root extract of Scutellaria baicalensis and its photocatalytic degradation activity using methylene blue. Optik (stuttg) 184:324–329. https://doi.org/10.1016/j.ijleo.2019.03.051
Darvishi E, Kahrizi D, Arkan E (2019) Comparison of different properties of zinc oxide nanoparticles synthesized by the green (using Juglans regia L. leaf extract) and chemical methods. J Mol Liq 286:110831. https://doi.org/10.1016/j.molliq.2019.04.108
Dash A, Ahmed MT, Selvaraj R (2019) Mesoporous magnetite nanoparticles synthesis using the Peltophorum pterocarpum pod extract, their antibacterial efficacy against pathogens and ability to remove a pollutant dye. J Mol Struct 1178:268–273
Diallo A, Ngom BD, Park E, Maaza M (2015) Green synthesis of ZnO nanoparticles by Aspalathus linearis: structural & optical properties. J Alloys Compd 646:425–430. https://doi.org/10.1016/j.jallcom.2015.05.242
Ekennia AC, Uduagwu DN, Nwaji NN, Oje OO, Emma-Uba CO, Mgbii SI, Olowo OJ, Nwanji OL (2020) Green synthesis of biogenic zinc oxide nanoflower as dual agent for photodegradation of an organic dye and tyrosinase inhibitor. J Inorg Organomet Polym Mater. https://doi.org/10.1007/s10904-020-01729-w
Ganesh M, Lee SG, Jayaprakash J, Mohankumar M, Jang HT (2019) Hydnocarpus alpina Wt extract mediated green synthesis of ZnO nanoparticle and screening of its anti-microbial, free radical scavenging, and photocatalytic activity. Biocatal Agric Biotechnol 19:101129. https://doi.org/10.1016/j.bcab.2019.101129
Gopalakrishnan Y, Tun U, Onn H, Al-gheethi A, Tun U, Onn H, Maya R, Radin S, Tun U, Onn H, Tun U, Onn H (2019) Green synthesis of ZnO nanoparticles by Coriandrum sativum leaf extract : structural and optical properties green synthesis of ZnO nanoparticles by Coriandrum sativum leaf extract : structural and optical properties AQ1. Desalin Water Treat. https://doi.org/10.5004/dwt.2019.24584
Groiss S, Selvaraj R, Varadavenkatesan T, Vinayagam R (2017) Structural characterization, antibacterial and catalytic effect of iron oxide nanoparticles synthesised using the leaf extract of Cynometra ramiflora. J Mol Struct 1128:572–578. https://doi.org/10.1016/j.molstruc.2016.09.031
Hasan M, Altaf M, Zafar A, Hassan SG, Ali Z, Mustafa G, Munawar T, Saif MS, Tariq T, Iqbal F, Khan MW, Mahmood A, Mahmood N, Shu X (2021) Bioinspired synthesis of zinc oxide nano-flowers: a surface enhanced antibacterial and harvesting efficiency. Mater Sci Eng C 119:111280. https://doi.org/10.1016/j.msec.2020.111280
Haynes WM (2014) CRC handbook of chemistry and physics. CRC Press
Hu D, Si W, Qin W, Jiao J, Li X, Gu X, Hao Y (2019) Cucurbita pepo leaf extract induced synthesis of zinc oxide nanoparticles, characterization for the treatment of femoral fracture. J Photochem Photobiol B Biol 195:12–16. https://doi.org/10.1016/j.jphotobiol.2019.04.001
Krishnan BR, Selvakumar MRM, Sasikumar SKA, Geerthi DV (2020) A facile green approach of cone—like ZnO NSs synthesized via jatropha gossypifolia leaves extract for photocatalytic and biological activity. J Inorg Organomet Polym Mater. https://doi.org/10.1007/s10904-020-01576-9
Letsholathebe D, Thema FT, Mphale K, Maabong K, Maria Magdalane C (2020) Green synthesis of ZnO doped Moringa oleifera leaf extract using Titon yellow dye for photocatalytic applications. Today Proc Mater. https://doi.org/10.1016/j.matpr.2020.05.119
Li JF, Rupa EJ, Hurh J, Huo Y, Chen L, Han Y, Chan Ahn J, Park JK, Lee HA, Mathiyalagan R, Yang D-C (2019) Cordyceps militaris fungus mediated zinc oxide nanoparticles for the photocatalytic degradation of methylene blue dye. Optik (stuttg) 183:691–697. https://doi.org/10.1016/j.ijleo.2019.02.081
Lu J, Batjikh I, Hurh J, Han Y, Ali H (2019) Photocatalytic degradation of methylene blue using biosynthesized zinc oxide nanoparticles from bark extract of Kalopanax septemlobus. Opt-Int J Light Electron Opt 182:980–985. https://doi.org/10.1016/j.ijleo.2018.12.016
Luo J, Ma SY, Sun AM, Cheng L, Yang GJ, Wang T, Li WQ, Li XB, Mao YZ, GZ DJ (2014) Ethanol sensing enhancement by optimizing ZnO nanostructure: From 1D nanorods to 3D nanoflower. Mater Lett 137:17–20. https://doi.org/10.1016/j.matlet.2014.08.108
Mirgane N, Shivankar V, Kotwal S, Wadhawa G, Sonawale M (2020) Degradation of dyes using biologically synthesized zinc oxide nanoparticles. Mater Today Proc. https://doi.org/10.1016/j.matpr.2020.06.037
Mirzaei H, Darroudi M (2017) Zinc oxide nanoparticles: biological synthesis and biomedical applications. Ceram Int 43:907–914
Nava OJ, Luque PA, Gómez-Gutiérrez CM, Vilchis-Nestor AR, Castro-Beltrán A, Mota-González ML, Olivas A (2017) Influence of Camellia sinensis extract on zinc oxide nanoparticle green synthesis. J Mol Struct 1134:121–125. https://doi.org/10.1016/j.molstruc.2016.12.069
Pai S, H S, Varadavenkatesan T, Vinayagam R, Selvaraj R (2019a) Photocatalytic zinc oxide nanoparticles synthesis using Peltophorum pterocarpum leaf extract and their characterization. Optik (stuttg) 185:248–255. https://doi.org/10.1016/j.ijleo.2019.03.101
Pandey S (2017) A comprehensive review on recent developments in bentonite-based materials used as adsorbents for wastewater treatment. J Mol Liq 241:1091–1113. https://doi.org/10.1016/j.molliq.2017.06.115
Pandey S, Do JY, Kim J, Kang M (2020a) Fast and highly efficient catalytic degradation of dyes using κ-carrageenan stabilized silver nanoparticles nanocatalyst. Carbohydr Polym 230:115597. https://doi.org/10.1016/j.carbpol.2019.115597
Pandey S, Do JY, Kim J, Kang M (2020b) Fast and highly efficient removal of dye from aqueous solution using natural locust bean gum based hydrogels as adsorbent. Int J Biol Macromol 143:60–75. https://doi.org/10.1016/j.ijbiomac.2019.12.002
Pandimurugan R, Thambidurai S (2016) Novel seaweed capped ZnO nanoparticles for effective dye photodegradation and antibacterial activity. Adv Powder Technol 27:1062–1072
Pudukudy M, Yaakob Z (2015) Facile synthesis of quasi spherical ZnO nanoparticles with excellent photocatalytic activity. J Clust Sci. https://doi.org/10.1007/s10876-014-0806-1
Raja S, Ramesh V, Thivaharan V (2015a) Antibacterial and anticoagulant activity of silver nanoparticles synthesised from a novel source–pods of Peltophorum pterocarpum. J Ind Eng Chem 29:257–264. https://doi.org/10.1016/j.jiec.2015.03.033
Raja S, Ramesh V, Thivaharan V (2015b) Antibacterial and anticoagulant activity of silver nanoparticles synthesised from a novel source-pods of Peltophorum pterocarpum. J Ind Eng Chem 29:257–264. https://doi.org/10.1016/j.jiec.2015.03.033
Tabrizi Hafez Moghaddas SM, Elahi B, Darroudi M, Javanbakht V (2019) Green synthesis of hexagonal-shaped zinc oxide nanosheets using mucilage from flaxseed for removal of methylene blue from aqueous solution. J Mol Liq 296:111834. https://doi.org/10.1016/j.molliq.2019.111834
Shim YJ, Soshnikova V, Anandapadmanaban G, Mathiyalagan R, Jimenez Perez ZE, Markus J, Ju Kim Y, Castro-Aceituno V, Yang DC (2019) Zinc oxide nanoparticles synthesized by Suaeda japonica Makino and their photocatalytic degradation of methylene blue. Optik (stuttg). 182:1015–1020. https://doi.org/10.1016/j.ijleo.2018.11.144
Soto-Robles CA, Nava O, Cornejo L, Lugo-Medina E, Vilchis-Nestor AR, Castro-Beltrán A, Luque PA (2021) Biosynthesis, characterization and photocatalytic activity of ZnO nanoparticles using extracts of Justicia spicigera for the degradation of methylene blue. J Mol StrUct. https://doi.org/10.1016/j.molstruc.2020.129101
Suresh D, Nethravathi PC, Rajanaika H, Nagabhushana H, Sharma SC (2015) others, Green synthesis of multifunctional zinc oxide (ZnO) nanoparticles using Cassia fistula plant extract and their photodegradative, antioxidant and antibacterial activities. Mater Sci Semicond Process 31:446–454
Suresh J, Pradheesh G, Alexramani V (2018) Green synthesis and characterization of zinc oxide nanoparticle using insulin plant (Costus pictus. D Don ) and investigation of its antimicrobial as well as anticancer activities. Adv Nat Sci Nanosci Nanotechnol 9:015008
Tamuly C, Saikia I, Hazarika M, Bordoloi M, Hussain N, Das MR, Deka K (2015) Bio-derived ZnO nanoflower: a highly efficient catalyst for the synthesis of chalcone derivatives. RSC Adv 5:8604–8608
Tripathi RM, Bhadwal AS, Gupta RK, Singh P, Shrivastav A, Shrivastav BR (2014) ZnO nanoflowers: novel biogenic synthesis and enhanced photocatalytic activity. J Photochem Photobiol B Biol 141:288–295
Varadavenkatesan T, Lyubchik E, Pai S, Pugazhendhi A, Vinayagam R, Selvaraj R (2019) Photocatalytic degradation of Rhodamine B by zinc oxide nanoparticles synthesized using the leaf extract of Cyanometra ramiflora. J Photochem Photobiol B Biol 199:111621. https://doi.org/10.1016/j.jphotobiol.2019.111621
Varadavenkatesan T, Selvaraj R, Vinayagam R (2020a) Green synthesis of silver nanoparticles using Thunbergia grandiflora flower extract and its catalytic action in reduction of Congo red dye. Mater Today Proc 23:39–42. https://doi.org/10.1016/j.matpr.2019.05.441
Varadavenkatesan T, Vinayagam R, Selvaraj R (2020b) Green synthesis and structural characterization of silver nanoparticles synthesized using the pod extract of Clitoria ternatea and its application towards dye degradation. Mater Today Proc 23:27–29. https://doi.org/10.1016/j.matpr.2019.04.216
Vinayagam R, Pai S, Varadavenkatesan T (2020a) Structural characterization of green synthesized α -Fe 2 O 3 nanoparticles using the leaf extract of Spondias dulcis. Surf Interfaces 20:1–9. https://doi.org/10.1016/j.surfin.2020.100618
Vinayagam R, Selvaraj R, Arivalagan P, Varadavenkatesan T (2020b) Synthesis, characterization and photocatalytic dye degradation capability of Calliandra haematocephala-mediated zinc oxide nanoflowers. J Photochem Photobiol B Biol 203:111760. https://doi.org/10.1016/j.jphotobiol.2019.111760
Acknowledgements
The authors are grateful to the Department of Chemical Engineering, Manipal Institute of Technology, Manipal Academy of Higher Education for providing lab facilities and equipment to perform this study. The authors thank DST PURSE Laboratory, Mangalore University, Mangalagangotri for providing the FE-SEM and EDS facilities.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vinayagam, R., Pai, S., Murugesan, G. et al. Synthesis of photocatalytic zinc oxide nanoflowers using Peltophorum pterocarpum pod extract and their characterization. Appl Nanosci 13, 847–857 (2023). https://doi.org/10.1007/s13204-021-01919-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-021-01919-z