Abstract
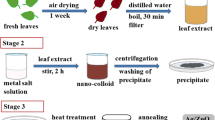
Here, we report for the first time the synthesis of new composite Ag-NPs-loaded core/shell mixed oxides NPs, CdO/Co3O4. First, nano-precursors (1) and (2) consist of mixed (cadmium oxalate/cobalt oxalate) and (silver oxalate/cadmium oxalate/cobalt oxalate), respectively, have been synthesized and characterized. Then, thermal treatment of 1 and 2 at 400 °C resulted in the formation of composites CdO/Co3O4 (3) and Ag-NPs@CdO/Co3O4 (4), respectively. The materials have been characterized by IR, SEM, TEM, TGA and XRD. The morphologies of the materials were described from SEM images. TEM images revealing the formation of core–shell structure with average particles size 5–20 nm. The crystal sizes were calculated from XRD patterns and resulted in 14.21, 14.65, 15.06 and 15.79 nm for 1, 2, 3 and 4, respectively. All the materials undergo tests as antibacterial agents against Gram-positive bacteria (Bacillus subtilis and Enterococcus spp.) and Gram-negative bacteria (E. coli and Pseudomonas aeruginosa). Precursor 2 and composite 4 impart higher inhibition against all the bacterial strains compared to precursor 1 and composite 3.










Similar content being viewed by others
References
Abo Zeid EF, Ibrahem IA, Ali AM, Mohamed WA (2019) The effect of CdO content on the crystal structure, surface morphology, optical properties and photocatalytic efficiency of p-NiO/n-CdO nanocomposite. Results in Physics 12:562–570
Abo Zeid EF, Nassar AM, Hussein MA, Alam MM, Asiri AM, Hegazy HH, Rahman MM (2020) Mixed oxides CuO-NiO fabricated for selective detection of 2-aminophenol by electrochemical approach. J Mater Res Technol 9(2):1457–1467
Abureesh MA, Oladipo AA, Mizwari ZM, Berksel E (2018) Engineered mixed oxide-based polymeric composites for enhanced antimicrobial activity and sustained release of antiretroviral drug. Int J Biol Macromol 116:417–425
Alsohaimi IH, Nassar AM, Seaf Elnasr TA, Cheba BA (2020) A novel composite silver nanoparticles loaded calcium oxide stemming from egg shell recycling: A potent photocatalytic and antibacterial activities. J. Clen Prod 248:119274
Assim K, Schulze S, Pügner M, Uhlemann M, Gemming T, Giebeler L, Lang H (2017) Co (II) ethylene glycol carboxylates for Co3O4 nanoparticle and nanocomposite formation. J Mater Sci 52(11):6697–6711
Balamurugan S, Balu AR, Usharani K, Suganya M, Anitha S, Prabha D, Ilangovan S (2016) Synthesis of CdO nanopowders by a simple soft chemical method and evaluation of their antimicrobial activities. Pacific Sci Rev A: Nat Sci Eng 18(3):228–232
Basu T, Ghosh UC (2013) Nano-structured iron (III)–cerium (IV) mixed oxide: synthesis, characterization and arsenic sorption kinetics in the presence of co-existing ions aiming to apply for high arsenic groundwater treatment. Appl Surf Sci 283:471–481
Bayal N, Jeevanandam P (2012) Synthesis of CuO@ NiO core-shell nanoparticles by homogeneous precipitation method. J Alloy Compd 537:232–241
Du H, Wang J, Wang B, Cang D (2010) Preparation of cobalt oxalate powders with the presence of a pulsed electromagnetic field. Powder Technol 199(2):149–153
Elango M, Deepa M, Subramanian R, Saraswathy G (2018) Synthesis, structural characterization and antimicrobial activities of polyindole stabilized Ag-Co3O4 nanocomposite by reflux condensation method. Mater Chem Phys 216:305–315
Elseman AM, Shalan AE, Rashad MM, Hassan AM, Ibrahim NM, Nassar AM (2017) Easily attainable new approach to mass yield ferrocenyl Schiff base and different metal complexes of ferrocenyl Schiff base through convenient ultrasonication-solvothermal method. J Phys Org Chem 30(6):e3639
Fouad OA, Makhlouf SA, Ali GA, El-Sayed AY (2011) Cobalt/silica nanocomposite via thermal calcination-reduction of gel precursors. Mater Chem Phys 128(1–2):70–76
Gomes GA, da Costa GL, da Silva Figueiredo ABH (2018) Synthesis of ferrite nanoparticles Cu1− xAgxFe2O4 and evaluation of potential antibacterial activity. J Mater Res Technol 7(3):381–386
Gong P, Li H, He X, Wang K, Hu J, Tan W, Yang X (2007) Preparation and antibacterial activity of Fe3O4@ Ag nanoparticles. Nanotechnology 18(28):285604
Koohestani H (2019) Characterization of TiO2/WO3 composite produced with recycled WO3 nanoparticles from WNiFe alloy. Mater Chem Phys 229:251–256
Kooti M, Saiahi S, Motamedi H (2013) Fabrication of silver-coated cobalt ferrite nanocomposite and the study of its antibacterial activity. J Magn Magn Mater 333:138–143
Li Y, Tan B, Wu Y (2008) Mesoporous Co3O4 nanowire arrays for lithium ion batteries with high capacity and rate capability. Nano Lett 8(1):265–270
Li X, Zhu Z, Zhao Q, Wang L (2011) Photocatalytic degradation of gaseous toluene over ZnAl2O4 prepared by different methods: a comparative study. J Hazard Mater 186(2–3):2089–2096
Liu J, Wang Z, Luo Z, Bashir S (2013) Effective bactericidal performance of silver-decorated titania nano-composites. Dalton Trans 42(6):2158–2166
Makhlouf SA, Bakr ZH, Aly KI, Moustafa MS (2013) Structural, electrical and optical properties of Co3O4 nanoparticles. Superlattices Microstruct 64:107–117
Małecka B, Łącz A (2008) Thermal decomposition of cadmium formate in inert and oxidative atmosphere. Thermochim Acta 479(1–2):12–16
Manjula N, Pugalenthi M, Nagarethinam VS, Usharani K, Balu AR (2015) Effect of doping concentration on the structural, morphological, optical and electrical properties of Mn-doped CdO thin films. Mater Sci-Poland 33(4):774–781
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16(10):2346
Nassar AM, Zeid EA, Elseman AM, Alotaibi NF (2018) A novel heterometallic compound for design and study of electrical properties of silver nanoparticles-decorated lead compounds. New J Chem 42(2):1387–1395
Nassar AM, Elseman AM, Alsohaimi IH, Alotaibi NF, Khan A (2019) Diaqua oxalato strontium (II) complex as a precursor for facile fabrication of Ag-NPs@ SrCO3, characterization, optical properties, morphological studies and adsorption efficiency. J Coord Chem 72(5–7):771–785
Nayaka GP, Pai KV, Santhosh G, Manjanna J (2016) Recovery of cobalt as cobalt oxalate from spent lithium ion batteries by using glycine as leaching agent. J Environ Chem Eng 4(2):2378–2383
Perez C (1990) Antibiotic assay by agar-well diffusion method. Acta Biol Med Exp 15:113–115
Rai MK, Deshmukh SD, Ingle AP, Gade AK (2012) Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J Appl Microbiol 112(5):841–852
Raj AME, Jayanthi DD, Jothy VB (2008) Optimized growth and characterization of cadmium oxalate single crystals in silica gel. Solid State Sci 10(5):557–562
Ren YY, Yang H, Wang T, Wang C (2019) Bio-synthesis of silver nanoparticles with antibacterial activity. Mater Chem Phys 235:121746
Sadhukhan S, Ghosh TK, Roy I, Rana D, Bhattacharyya A, Saha R, Chattopadhyay D (2019) Green synthesis of cadmium oxide decorated reduced graphene oxide nanocomposites and its electrical and antibacterial properties. Mater Sci Eng, C 99:696–709
Sakthivel P, Asaithambi S, Karuppaiah M, Yuvakkumar R, Hayakawa Y, Ravi G (2020) Improved optoelectronic properties of Gd doped cadmium oxide thin films through optimized film thickness for alternative TCO applications. J Alloy Compd 820:153188
Salam MA, Abu Khadra MR, Mohamed AS (2020) Effective oxidation of methyl parathion pesticide in water over recycled glass based-MCM-41 decorated by green Co3O4 nanoparticles. Environ Pollut 259:113874
Salem A (2014) Silver-doped cadmium oxide nanoparticles: synthesis, structural and optical properties. The Euro Physical J Plus 129(12):1–12
Sun L, Qiu K (2012) Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries. Waste Manage 32(8):1575–1582
Usharani K, Balu AR, Nagarethinam VS, Suganya M (2015) Characteristic analysis on the physical properties of nanostructured Mg-doped CdO thin films—doping concentration effect. Progress Nat Sci: Mater Int 25(3):251–257
Vennela AB, Mangalaraj D, Muthukumarasamy N, Agilan S, Hemalatha KV (2019) Structural and optical properties of Co3O4 nanoparticles prepared by sol-gel technique for photocatalytic application. Int J Electrochem Sci 14:3535–3552
Wang X, Yang Y, Zhang F, Tang J, Guo Z (2020) Facile synthesis of Co3O4/CdO nanospheres as high rate performance supercapacitors. Mater Lett 261:127141
Williams JM, Beno MA, Carlson KD, Geiser U, Kao HI, Kini AM, Thorn RJ (1988) High transition temperature inorganic oxide superconductors: synthesis, crystal structure, electrical properties, and electronic structure. Acc Chem Res 21(1):1–7
Yang W, Wang C, Arrighi V (2018) Silver oxalate ink with low sintering temperature and good electrical property. J Electron Mater 47(5):2824–2835
Yuan C, Wu HB, Xie Y, Lou XW (2014) Mixed transition-metal oxides: design, synthesis, and energy-related applications. Angew Chem Int Ed 53(6):1488–1504
Zahera M, Khan SA, Khan IA, Sharma RK, Sinha N, Al-Shwaiman HA, Khan MS (2020) Cadmium oxide nanoparticles: an attractive candidate for novel therapeutic approaches. Colloids Surf, A 585:124017
Zakrzewska K (2001) Mixed oxides as gas sensors. Thin Solid Films 391(2):229–238
Zou D, Xu C, Luo H, Wang L, Ying T (2008) Synthesis of Co3O4 nanoparticles via an ionic liquid-assisted methodology at room temperature. Mater Lett 62(12–13):1976–1978
Acknowledgment
The authors are thankful for Jouf University for supporting this work by chemicals, laboratories, and analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
No competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nassar, A.M., Alrowaili, Z.A., Ahmed, A.A.M. et al. Facile synthesis of new composite, Ag-Nps-loaded core/shell CdO/Co3O4 NPs, characterization and excellent performance in antibacterial activity. Appl Nanosci 11, 419–428 (2021). https://doi.org/10.1007/s13204-020-01606-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-020-01606-5