Abstract
In this paper, superfine SiO2 nanoparticles are utilized as composite abrasive additive to improve the polishing property of CeO2, which is prepared through chemical precipitation method by rare-earth chloride as raw materials, silicofluoric acid as fluoride, Na2CO3 as modifier, and NH4HCO3 as precipitator. XRD, SEM, particle size analyzer, and atomic force microscopy are applied to characterize the physical structure, morphology, and size distribution. Furthermore, the as-prepared composite abrasive is used to perform chemical mechanical polishing (CMP) experiment on K9 glass to identify the impact of different mass fraction of SiO2 on the CMP property of work materials. The results show that the composite abrasive contained 0.5% SiO2 has the best polishing property, the surface roughness, and material removal rate of glass after polishing can reach at 1.3157 nm and 22.6 nm/min, respectively.
Similar content being viewed by others
Introduction
CeO2 is an important rare-earth oxide and has always been used to polish hard materials such as glass substrate with high hardness, though CeO2 itself has relatively low Moh’s hardness (6–7) (Belkhir et al. 2009; Savio et al. 2009; Chen et al. 2010). Researches show that compare with other traditional inorganic abrasives (such as SiO2, ZrO2, and A12O3), CeO2 is more suitable for conduct CMP on glass substrates with several advantages including high polishing rate and good polishing quality (Armini et al. 2007; Chen et al. 2015; Lu et al. 2011). In recent years, as the development of nanotechnology, the merits of small size and distinctive surface-interface effect owned by nanoparticles endow corresponding composite abrasives with better polishing properties. Meanwhile, the grinding speed and surface quality in the polishing process are greatly improved, which in turn decrease the related cost (Gao 2016). With the advancement of modern science and technology, requirements for the polishing process on specially used glasses are becoming increasingly higher. Therefore, the development of high-property composite abrasives and their application in CMP have gradually become a research hotspot in CMP related fields.
To enhance the quality and efficiency of wafer CMP, Zhang et al. (2014) prepared a green waterborne slurry through homogeneous precipitation by CeO2/SiO2 composite abrasive and then investigated its polishing property on silicon wafers, the results showed that as the pH value of polishing slurry increased, the removal rate of materials decreased with time. With the increment of polishing rate and polishing pressure, the removal rate of materials increased first then decreased. Shi et al. (2013) used SiO2 power, cerous nitrate, and ammonium bicarbonate as raw materials to prepare coated CeO2 @ SiO2 coated composite abrasive through chemical precipitation method, then applied it to polish K9 glass, and investigated the morphology differences of glass before and after polishing. The results showed that after polishing, number of scratches on glass surface decreased; meanwhile, RMS decreased from 54.217 to 1.073 nm. Chen et al. (2014) designed and prepared PMMA/CeO2 composite abrasive with controlled inorganic shell coating quantities by chemical in situ coating technology based on electrostatic interaction between positive and negative charges. The results of polishing test indicated that the coating quantity of CeO2 had much effect on the surface quality of the substrate; furthermore, ultra-smooth machined surface could be obtained after polishing. In addition, the average value of surface roughness (Ra) decreased from 0.63 ± 0.07 to 0.25 ± 0.01 nm. Chai and Zhou (2011) synthesized SiO2–CeO2 composite oxide, then utilized it to polish silicon wafers, and identified the relationship between polishing rate and different preparation conditions. The test results showed that after calcination at 800 °C, the composite oxide with molar ratio of 2:1 (silicon to cerium) had the largest polishing rate. Shi (2013) synthesized Ti-doped CeO2 composite abrasive via coprecipitation method, and then, investigated the impact of doping content and calcination temperature on properties of composite abrasive. From the results, it could be seen that after doping with Ti, CeO2 particles gradually shaped into spheres, the uniformity of particles improved, and the agglomeration had been obviously alleviated. The highest degree of crystallinity and largest polishing rate were realized at Ti content of 0.2 and calcination temperature of 900 °C.
Based on the current situation of CeO2 being the most widely applied rare-earth polishing powder, the author prepares superfine CeO2 abrasive for optical glass polishing; meanwhile, the composite abrasive is further prepared by adding SiO2 nanoparticles with different mass fractions. Particle size analyzer, potential instrument, SEM, and TEM are used for characterization of CeO2 and SiO2 nanoparticles. After that, polishing slurry made of the as-synthesized composite abrasive is prepared and its impact on the CMP property to K9 glass is investigated. After the polishing process, AFM is utilized to observe the surface morphology of glass; the surface roughness is measured, too.
Experiment
Preparation of CeO2
Rare-earth chloride and 5% NaOH (mass fraction) were first dissolved by industrial pure water, then 30% HCl (mass fraction) was added, and the mixture was stirred. In the stirring process, the pH of solution should be kept at 3.5–4, the concentration of rare-earth oxide (REO) should be maintained at 120–160 g/L. After stirring, the mixture was filtered and implanted into parent compound reactor; meanwhile, silicofluoric acid with REO concentration of 6.5–8% was added; in this process, the temperature should be kept at 40–80 °C. When the silicofluoric acid was uniformly dispersed in the reactor, 5% NaOH (mass fraction) and 97% C2H6O (mass fraction) were added into the reactor drop by drop to increase the pH of reactant inside up to 6–7. At this point, chlorinated lattice began to change, then continued to heat by steam, and maintained the temperature at 80–100 °C. 4–6 h later, the reactant was dewatered by a pressure filter and delivered to a sagger, and then be calcinated at 800 °C for 2 h to form into blocks. After natural cooling, the reactant was transferred to a dry sieving machine, and then smashed by a fluidized bed jet mill to obtain rare CeO2 particles. Figure 1 shows the flow diagram.
Characterization of CeO2 and SiO2 particles
Granularity analysis on CeO2 particles was performed by a BT-9300S laser granulometer from Dandong Baite Corporation. Size analysis on SiO2 particles was conducted by an SZ-100 particle analyzer from HORIBA. Small angle diffraction (XRD) analysis was carried out by a D8 Advance powder X-ray diffractometer from Bruker. Transmission electron microscope (TEM) and scanning electron microscope (SEM) were used to characterize the morphology and structure. In addition, HORIBA-SZ-100 particle analyzer was also used to measure the zeta potential value of SiO2 nanoparticles and the potential value of CeO2 and SiO2 particles.
Polishing experiment and evaluation
First, 150 g SiO2 nanoparticles and CeO2 polishing powder of different ratios was weighed by a chemical balance and placed in a beaker. Then, 0.2% dispersant was weighed by an electronic balance and added into. After that, added 500 mL deionized water was added, the mixture was stirred with a glass rod to obtain a uniform mixture of CeO2 and SiO2. Then, the mixture should be manually stirred for 20 min under water-bath heating and ultrasonic conditions. The mixed abrasives should be transferred to a container of no less than 4 L, the beaker was rinsed at least three times, and cleaning solution should be transferred the container, too. Industrial pure water was continuously added into the beaker until the volume of mixture reached to 3 L, and then, the mixture was stirred again and obtained solution with uniformly dispersed additive particles was finally obtained. The final solution was used to prepare polishing slurries; after being ultrasonically dispersed for 15 min, slurries were tested by a glass polishing tester.
Before testing, the weight of each glass sample was measured by a one over ten-thousand electronic balance before testing. When the test began, the water suction pump should be activated first to let the polishing solution circulated inside the tester for 2–3 min. After the polishing solution being fully distributed on the glass plate and polishing plate, the glass plate was erected and three K9 glasses were embedded into three circular recesses in the glass plate (Fig. 2). Then, the glass plate should be laid down slowly, the polishing time was set as 30 min, and test temperature was 20 °C.
After the test, plenty of industrial pure water was used to rinse the tester, polishing plate, and containers for store polishing storage. Then, K9 glass samples were took out, dried, weighed, and the weight difference before and after the test was calculated. The polishing rate (MRR) of polishing abrasives is presented by the cutting thickness of the polished glass surface per unit time, the detailed measuring methods are as follows: first, subtraction method is used to determine the volume of etching before and after polishing and the figure out; the volume of etching on glass surface per unit is calculated, and finally, it should be converted into the cutting thickness per unit time according to the glass density. The roughness and polishing rate in this paper were average values of three measurements. The morphology of glass surface after polishing was investigated by Nanite AFM from Nanosurf, the build-in analysis software was utilized to calculate the surface roughness and plot micro-profile curve, and the scan range was 10 μm × 10 μm.
Results and discussion
Particle size analysis
Figures 3, 4 show the size distribution curve of CeO2 and SiO2 particles, respectively. According to Fig. 3, most CeO2 particles have the size of < 8 μm. The curve marked by blue squares represents the possible percentage of particles with size smaller than a certain value, which shows that 95% CeO2 particles have the size of < 10 μm, further proves the relative stable size distribution. Figure 4 shows that most SiO2 powders have the size of 250–500 nm. Similar to the above one, the curve marked by blue squares also represents for the possible percentage of particles with size smaller than a certain value. In this case, all SiO2 particles have size smaller than 500 nm, which demonstrates the small size of selected nanoparticles and the excellent stable size distribution. To sum up, when blending SiO2 nanoparticles with CeO2, because of the high stability of both particles, they will not experience large morphological changes in the polishing process. Therefore, the polishing efficiency and surface polishing quality can be greatly improved.
XRD analysis
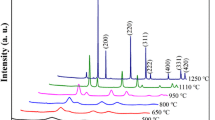
Figure 5b shows the XRD spectra of prepared sample and Fig. 5a shows the XRD spectra of the standard card of CeO2. It can be seen that the position and relative intensity of characteristic diffraction peaks (2θ = 28.8°, 33.1°, 48.1° and 56.8°) of prepared composite particle are basically consistent with the standard card of CeO2 (JCPDS card, No. 34-0394). According to the position, each peak corresponds to (111), (200), (220), and (311) crystal face, respectively. In addition, sharp peaks represent for (400), (331), and (420) crystal face are also easy to identify and show high diffraction intensities, which prove good crystal form of the sample. The prepared sample shows high purity, since no diffraction peak corresponds to other materials found in the XRD spectra. Compare with the standard card of CeO2 on the left side of Fig. 5a, it is found that the prepared sample has narrower half-peak width at each corresponding position, which demonstrates smaller average grain size. Figure 6 shows that no apparent peak (crest and trough) is found in the XRD spectra of SiO2 nanoparticle. Therefore, in general, SiO2 nanoparticles are considered as amorphous materials.
SEM and TEM characterization
SEM and TEM are used to investigate the morphology of randomly selected rare-earth CeO2 and SiO2 after preparation. The SEM and TEM of CeO2 particles are shown in Fig. 7a, b, respectively. CeO2 particles are irregular shape and the size of CeO2 particles < 10 μm. The SEM and TEM of SiO2 particles are shown in Fig. 7c, d, respectively. The results show that the SiO2 particles are spherical; the distribution is more uniform, the diameter of the SiO2 ball is about 200–500 nm. Through a series of processing, no obvious agglomeration is observed, the calcinated product get a loose structure made up of many small particles, of which monodispersed particles with loose structure and uniform distribution could be clearly identified. The reason behind this is that the undispersed particles are completely smashed by the jet mill, which facilitates the production of small CeO2 particles with uniform size. The uniform size and stable dispersion property help the dispersion of the two particles in polishing solution more easily and prevent them from agglomeration, which play an important role in the improvement of polishing property.
Measurement and analysis of zeta potential
ZETA potential has great significance, since it is closely related to colloidal dispersion stability. In general, higher zeta potential (positive or negative) leads to higher system stability; otherwise, lower potential results in a more volatile system.
According to Table 1, and Figs. 8 and 9, the zeta potential of SiO2 nanoparticles ranges from ± 40 to ± 60, which shows the good stability; while the zeta potential of CeO2 ranges from ± 10 to ± 30, which indicates relatively poor stability. On the other hand, since both materials have negative potential, agglomeration caused by opposite charges can be well prevented in the blending process. Therefore, these two kinds of powders can be blended with any ratio to realize better grinding effect.
Polishing property of the composite abrasive
Table 2 lists the sample code of CeO2 composite abrasives with different SiO2 content. Figure 10 shows the AFM pattern of K9 glass polished by A0, A1, A2, and A3. For the AFM hypsogram, areas with lighter color represent higher positions, while dark areas indicate lower positions; for the 2D topography, various colored columns reflect the relationship between color difference and relative height. Figure 10(A0) shows that after polished by CeO2 abrasive without SiO2, rough surface was formed which contains obvious ups and downs, the average value of RMS is calculated as 3.8913 nm in the field of 10 μm × 10 μm. The planarization degree of K9 glass experiences great improvement [Fig. 10(A1), (A2), (A3)] after being polished by CeO2 composite abrasives contained 0.3, 0.5, and 0.8% SiO2. Meanwhile, a flattening trend could be observed from 3D topography figures, and the RMS value is calculated to be 1.6085, 1.3157, and 1.4756 nm, respectively. The polishing rate MRR of CeO2 composite abrasives contained 0, 0.3, 0.5, and 0.8% SiO2 is calculated as 20.7, 20.3, 22.6, and 22.2 nm/min, respectively. In conclusion, the K9 glass being polished by CeO2 composite abrasive contained 0.5% SiO2 shows the smoothest surface and most uniform color distribution in the AFM pattern; no obvious height difference is observed. In addition, the RMS and MRR value of polished glass reaches at 1.3157 nm and 22.6 nm/min, respectively. Furthermore, this composite abrasive has the highest polishing rate compares to other three candidates.
To better reflect the smoothness of substrate surface before and after polishing, corresponding micro-profile analysis was performed by selecting a diagonal of AFM 2D topography (the black dotted line shown in Fig. 10) as the measurement position, which is shown in Fig. 11. It can be seen from Fig. 10(A0), (A1), and (A3) that the surface profile of original glass polished by CeO2 composite abrasive contained 0, 0.3 and 0.8% SiO2 fluctuates in the range of + 20.6–32.4 nm, + 11.3–13.5 nm, and + 10.1–7.03 nm, respectively. While according to Fig. 10(A2), the surface profile of original glass polished by CeO2 composite abrasive contained 0.5% SiO2 changes within + 7.6–9.28 nm. Under the same test conditions, AFM pattern and surface profile analyses all indicate that CeO2 composite abrasive contained 0.5% SiO2 can effectively improve the smoothness and decrease the surface roughness of polished K9 glass. What is more, the prepared composite abrasive can significantly increase the polishing rate on K9 glass, which indicates the great potential of these materials in polishing applications demand for high efficiency and zero damage. Furthermore, in the CMP process, part of the atomic layers on the surface of SiO2 nanoparticles will be dissolved by the polishing solution, which leads to the breakdown of internal Si–O–Si bond and formation of new Si–O bond. Meanwhile, self-ionized hydrogen ion in the polishing solution facilitates the formation of hydroxyl radicals (–OH) by the reaction of SiO2 nanoparticles and water. At this point, the Si–O bond will convert into Si–OH bond with relative low bond energy, which will finally convert into soft Si(OH)4 layer (Fig. 12). It is believed that this hydration reaction on SiO2 surface decreases its hardness and mechanical strength. Moreover, heat is generated on SiO2 surface through friction in the CMP process, which provides energy for the breakdown of Si–O bond and lowers the hardness of SiO2. Therefore, the addition of SiO2 particles can not only effectively reduce the number of scratches on glass surface in the CMP process, but also further restore the surface and improve the smoothness after polishing. The hydration reaction between the surface atomic layer of SiO2 and water molecules is shown as follows:
Conclusion
-
1.
The author carried out studies on the property of rare-earth abrasives, which further prepared a series of superfine cerium-silicon composite oxide for optical glass polishing through different processes.
-
2.
According to the size distribution curve of cerium oxide and silicon dioxide particles in the revised manuscript, we know that the particle size of rare-earth cerium oxide and nano-silica is still relatively stable. As shown by the XRD pattern, the crystalline form of rare-earth cerium oxide is good nano-silica is amorphous. From the SEM and TEM images, we can see that there is no obvious reunion of rare-earth cerium oxide. The ZETA potential results show earth cerium oxide and nano-silica have a negative potential; at the same time, rare-earth cerium oxide particles stability, and the stability of nano-silica particles is very good.
-
3.
Polishing property of rare-earth abrasives could be promoted in various degrees by blending it with silicon dioxide nanoparticles. Tests show that the polishing solution contained 0.5% silicon dioxide nanoparticles has the best polishing properties, the roughness (RMS) value, and MRR of K9 glass surface after polishing reach at 1.3157 nm and 22.6 nm/min, respectively.
References
Armini S, Whelan CM, Maex K et al (2007) Composite polymer-core silica-shell abrasive particles during oxide CMP: a defectivity study. J Electrochem Soc 154(154):H667
Belkhir N, Bouzid D, Herold V (2009) Wear behavior of the abrasive grains used in optical glass polishing. J Mater Process Technol 209(20):6140–6145
Chai MX, Zhou XZ (2011) Evaluation of polishing property for silicon wafer of SiO2-CeO2 mixed oxides. J Chin Soc Rare Earths 29(3):365–370
Chen Y, Long RW, Chen ZG (2010) Preparation and polishing performance of CeO2@SiO2 composite abrasives. Mater Rev 24(18):35–38
Chen Y, Li ZN, Song ZT, Min GQ (2014) Effect of the core size of the core /shell structured PS/SiO2 composite abrasives on oxide-CMP behavior. J Funct Mater 45(19):19121–19127
Chen Y, Li Z, Miao N (2015) Polymethylmethacrylate (PMMA)/CeO2, Hybrid particles for enhanced chemical mechanical polishing performance. Tribol Int 82(82):211–217
Gao Q (2016) Polishing characteristics of fixed-abrasive pad by using nano-aggregate silica. Opt Precis Eng 24(10):2490–2497
Lu JX, Chen ZG, Long RW (2011) Preparation, characterization and performance of core-shell PS-CeO2 composite abrasives with different particle sizes. Chin J Nonferrous Metal 21(7):1777–1784
Savio G, Meneghello R, Concheri G (2009) A surface roughness predictive model in deterministic polishing of ground glass moulds. Int J Mach Tools Manuf 49(1):1–7
Shi YB (2013) The synthesis of cerium oxide based composite rare earth polishing powder and study of its performance. Dissertation, East China University of Science and Technology
Shi YB, Qiu-Fang WU, Yi H (2013) Preparation of SiO2@CeO2 composite abrasive and its polishing performance. Chin Rare Earths 34(1):53–58
Zhang Q, Huang G, Zhao Y (2014) Research on polishing performances and mechanisms of CeO2/ SiO2 composite abrasives. Lubr Eng 39(1):59–63
Acknowledgements
This research project was supported by the National Natural Science Foundation of China (51105002), the National Science and Technology Major Project (2012ZX04005-021), the Natural Science Foundation of the Henan Province (152102210196), and the Foundation of the Henan Educational Committee (16A460001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, Q. Experimental study on polishing performance of CeO2 and nano-SiO2 mixed abrasive. Appl Nanosci 8, 163–171 (2018). https://doi.org/10.1007/s13204-018-0657-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-018-0657-4