Abstract
With the quality of crude oil becoming worse, the efficient Fluid catalytic cracking (FCC) conversion of heavy oil is of great challenge. The enhancement of isomerization during catalytic cracking process is a feasible approach to improve the gasoline yield and quality. In this study, meso-SAPO-11 was synthesized by citric acid modification to generate mesopores in the SAPO-11 molecular sieve. The modification temperature played an important role in the mesopore generation. Nitrogen sorption and X-ray diffraction analysis had been utilized to characterize the mesoporous structure. Meso-SAPO-11 was further used as an additive in the FCC catalyst for catalytic evaluation with atmospheric gas oil and coking gas oil. The additive showed significant improvement of heavy oil conversion, especially for the gasoline yield and quality .
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the petroleum refinery industry of China, the most important unit is the Fluid catalytic cracking plant, which is the leading process with huge processing amount and considerable profit. To be noted that the FCC light products contribute to almost 80 % of vehicle use gasoline and 40 % of diesel, respectively [1–3]. However, with the crude oil becoming even heavier, the processing of heavy oil via FCC unit is meeting great challenge [4, 5]. The FCC conversion will transform the large carbon-chain molecules into small hydrocarbons, during which process the catalyst plays a significant role for this conversion. The hydrocarbons of light oil can be easily transferred and converted in the microporous zeolites, however the conversion of heavy oil with longer carbon chains and complicated composition to gasoline is not satisfactory, besides the quality of FCC gasoline is even poor with low octane number. Therefore, the development of FCC catalysts which can improve the conversion of heavy oil and produce more gasoline product of better quality is of great need.
Isomerization process is an important process converting n-alkanes to branched isomers. These isomers possess higher activity of secondary cracking and contribute to the higher octane numbers in gasoline. For this reason, by enhancing the isomerization reactions in FCC process, the heavy oil conversion and gasoline quality will see a great improvement. The typical catalyst for isomerization is microporous SAPO-11 molecular sieve, which has an AEL topology and one-dimensional pore structure. Since the first report of SAPO-11 molecular sieve [6–8], numerous reports have studied its isomerization conversion of hydrocarbons with different metal incorporation [9–11], surface modification [12] and size variation [13]. However, the micropore of SAPO-11 inhibits its application in long-chain paraffin isomerization, due to their difficulty of mass transfer and catalytic conversion inside the pores. For this reason, mesoporous SAPO-11 has been initiated for the long-chain alkane transformation [14, 15], however, conventional method of meso-SAPO-11 synthesis need expensive templates and can be hardly applied in scale. Therefore a facile method for meso-SAPO-11 synthesis is significant for adding it into FCC catalyst as additives to enhance the isomerization conversion.
Among the methods of generating mesopores in zeolites, post treatment, especially for acid treatment process, has been widely employed for USY and ZSM-5 modification [16, 17]. It has been demonstrated that the acid can etch some of the compositions in silica-aluminate zeolite and the mesopores will be generated during the etching process [18, 19]. Citric acid, though with moderate acidity, exhibited better performance in generating mesopores than that with strong acid like HCl [20].
Herein, we report a facile way for meso-SAPO-11 fabrication by citric acid (CA) modification and further employ it as additive into the typical FCC catalyst for catalytic activity evaluation with atmospheric gas oil (AGO) and Coking gas oil (CGO).
Experiment section
Synthesis of meso-SAPO-11
Microporous SAPO-11 molecular sieve was purchased from Nankai Catalyst Company. The meso-SAPO-11 catalyst was synthesized by the citric modification to generate mesopores.
Typically, 3 g of SAPO-11 molecular sieve was dispersed into 0.5 mol/L citric acid solution. The reactions were conducted at selected temperatures in the water bath for 30 min. The final suspension was filtered and washed with deionized water, then dried at 100 °C for 6 h and calcined at 550 °C to obtain the CA modified meso-SAPO-11.
Characterization methods
The crystallinity of the prepared SAPO-11 was analyzed by XRD, which was carried out on an X’Pert PROMPD (Netherlands, Cu target, Kα radiation λ = 0.1542 nm, 35 kV, 40 mA), using molecular sieve phase analysis method, scan angle of 5º–45º. N2 adsorption/desorption isotherms were recorded at 77 K with a Micromeritics TriStar 3000. The total surface areas of the samples were calculated by the BET method, the mesopore surface area and pore volume were calculated by the BJH method, and the pore size distributions were derived from the BJH desorption branches.
Catalytic cracking evaluation
Catalyst preparation: The FCC catalysts were prepared using typical USY zeolites as the active component with mass composition of 30 %, and as-synthesized meso-SAPO-11 was added (10 wt %) as additive. 50 wt % of kaolin and 10 wt % of silica gel were, respectively, utilized as the support and binding agent. All the components were stirred in water for 2 h and dried at 100 C for 3 h, followed by calcination at 700 °C for 2 h. The catalyst was finally obtained after the hydrothermal treatment at 760 °C for 4 h with 100 % of water. The catalyst was granules pressed to small pellets with sieve sizes of 80–180 mesh before the further catalytic activity evaluation.
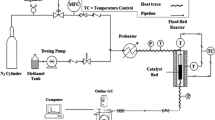
FCC evaluation: 3 g of the prepared catalyst was filled in a fixed-bed microreactor. The reaction was carried out at 500 °C and using 1.3 g of feed stock oil as feedstock. The products were collected before analysis. The conversion of heavy oil was defined as the weight percentage of feedstock converted to dry gas, liquid petroleum gas (LPG), gasoline, diesel and coke. Light oil is defined as the total amount of gasoline and diesel. The simplified scheme (Scheme 1) for micro activity tests is shown as below.
Results and discussion
Synthesis of meso-SAPO-11 by CA modification
Here, citric acid was chosen as the modification agent, which was used directly to etch the microporous SAPO-11 molecular sieves. In the modification process, the temperature is quite crucial since it is kinetically favorable of de-alumination in citric acid etching of alumina framework. Modification at different temperatures (23–80 °C) has been investigated to adjust the surface properties and pore structures of SAPO-11.
It can be seen from Table 1 that the surface area and pore volume of modified meso-SAPO-11 with citric acid at 23 °C did not change obviously. However at elevated temperatures, the surface areas and pore volumes of meso-SAPO-11 especially for the mesopores increased dramatically. This may result from the favorable de-alumination process at high temperature, which accelerate the speed of citric acid etching in SAPO-11 framework and generate more defects forming the mesopores.
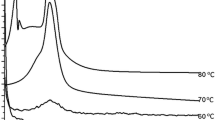
N2 adsorption/desorption isotherms of the CA modified meso-SAPO-11 at different temperature are given as Fig. 1. It can be identified from the isotherms that the samples were of mesopores with varied volume. At higher treatment temperature, the adsorption isotherms with P/P0 between 0.6 and 1.0 tend to be even steep, which indicated that more mesopores were generated at high temperature. With temperature higher than 50 °C, the hysteresis loop was broadened with H3 type, which indicated the obvious mesopores generated by crystals stacking and gaping.
As shown from the pore size distribution of the meso-SAPO-11 modified by CA at different temperatures (Fig. 2), with higher temperature more of the mesopores will be generated with relatively narrow pore size distribution. At the temperature higher than 40 °C, the mesopores centered at about 10 nm which were generated by the enhanced de-alumination at high temperature.
At the same time, the XRD patterns of the meso-SAPO-11 modified with CA at different temperature were characterized. As shown in Fig. 3, the characteristic peaks of AEL structure for SAPO-11 were quite obvious below 40 °C with citric acid treatment. However with modification at higher temperatures, the intensities of the characteristic peaks, especially for the peak around 21.2°, decreased dramatically, which was caused by the severe de-alumination on [002] facet of the framework in SAPO-11 [21]. The enhanced de-alumination at high temperature not only generated abundant mesopores, but also partly destroyed the crystal framework, and even etched most part of the SAPO-11 forming amorphous species at 80 °C.
Though higher temperature can generate more of the mesopores, the SAPO-11 crystals can hardly bear the severe etching and the frameworks get heavily damaged. Therefore, compromising the surface area, pore volume and its crystallinity, the modification temperature of 40 °C is relatively suitable for further application.
Preparation of FCC catalysts
In the fixed-bed microreactor for catalytic activity tests, the temperature was fixed at 500 °C, the feed stock injection amount was 1.3 g, and the catalyst-to-oil ratio is three. Three different FCC catalysts were prepared for comparison:
Cat1: 30 % USY + 60 % Kaolin + 10 % Silica gel;
Cat2: 30 % USY + 10 % microporous SAPO-11 + 50 % Kaolin + 10 % Silica gel;
Cat3: 30 % USY + 10 % meso-SAPO-11 + 50 % Kaolin + 10 % Silica gel;
The catalysts were hydrothermally treated at 760 °C for 4 h before usage. It is shown in Fig. 4 that the crystallinity of the molecular sieves in FCC catalysts decreased, however, the catalysts still retained the characteristic peaks for zeolite Y. Besides, there are characteristic peaks for SAPO-11 in Cat2 and Cat3, demonstrating the existence of SAPO-11 additives even after high-temperature hydrothermal treatment. With the crystals of SAPO-11 additives, it is meaningful to measure the catalytic activity of meso-SAPO-11 which may play a role for the FCC conversion. The results are discussed in later experiments.
Catalytic activity evaluations with AGO
The evaluations of three catalysts were conducted firstly using atmospheric gas oil (AGO), which had relatively better quality. For comparison of typical FCC catalyst Cat1, Cat2 with microporous SAPO-11 and Cat3 with meso-SAPO-11, the cracking conversion of AGO was studied and the isomerization was indicated by the isomers in gasoline product.
As shown in Table 2, the addition of SAPO-11 in Cat2 and meso-SAPO-11 in Cat3 increased the conversion of AGO dramatically, and the gasoline yield even nearly doubled. This may result from the increase of catalyst acidity with the addition of SAPO-11/meso-SAPO-11, which is favorable for large molecular cracking into gasoline fraction. By comparison of Cat2 and Cat3, the meso-structures in meso-SAPO-11 improved the cracking conversion of hydrocarbons with higher light oil yield by 5.58 % and less coke formation by 0.26 % compared with microporous SAPO-11. Meanwhile, the isomer selectivity in gasoline product with Cat3 also indicated better performance of meso-SAPO-11, possessing more than 90 % of isomers with the enhancement of isomerization conversion during the catalytic cracking process. This may be caused by the micro-mesoporous structure of meso-SAPO-11 in Cat3, which enable the large molecules easily to transfer inside the pores and undergo isomerization conversion on the active sites of SAPO-11. At the same time, the expedite channels for mass transfer also rendered the less contact time of the reactant on the acid sites, which decreased the possibility for secondary cracking and deep dehydrogenation to form coke.
Catalytic cracking evaluations with CGO
The FCC evaluation of three catalysts was conducted with coking gas oil (CGO), which had relatively worse quality of feedstock with larger hydrocarbon molecules and complicated composition. By comparison of typical FCC catalyst Cat1, Cat2 with microporous SAPO-11 and Cat3 with meso-SAPO-11, the cracking conversion of CGO was studied, and specifically the enhancement of isomerization was indicated by the isomers in gasoline product.
As shown in Table 3, compared with AGO, the conversion of CGO hardly reached 50 % with the gasoline and diesel yields becoming even lower. However, it is still quite obvious that the addition of SAPO-11 in Cat2 and meso-SAPO-11 in Cat3 increased the conversion of CGO and the gasoline yields nearly doubled compared with Cat1. For the comparison of catalysts with additives of microporous SAPO-11 and meso-SAPO-11, the latter seems to promote the cracking conversion of hydrocarbons. Meanwhile, the isomer selectivity in gasoline product also indicated a better performance for Cat3 with meso-SAPO-11, which increased by 2.13 % than Cat2 with microporous SAPO-11 during the catalytic cracking process. This may be caused by the meso-SAPO-11 as the additive in Cat3, enabling the large molecules easily transfer inside the pores and isomerize on the active sites of SAPO-11. During the FCC process, the introduction of mesopores in meso-SAPO-11 not only promoted the catalytic cracking of large hydrocarbons but also improved the isomer yields in the gasoline product which was crucial to improve the FCC gasoline quality.
Conclusion
With citric acid modification, meso-SAPO-11 can be achieved with mesopores centered at about 10 nm. By varying the treatment temperatures, the mesopores of meso-SAPO-11 can be controlled, however, at high temperature treatment, the crystallinity decrease resulted from the severe de-alumination process. The meso-SAPO-11 was used as an additive in the FCC catalyst for catalytic activity evaluation with two kinds of feedstock, atmospheric gas oil (AGO) and coking gas oil (CGO). The addition of the meso-SAPO-11 increased the catalytic conversion of the heavy oils and promoted the yields and qualities of gasoline products by improving the isomerization during catalytic cracking process. This modified meso-SAPO-11 additive has promising application for converting the heavy oil into gasoline with higher quality.
References
Shan H, Li C, Niu G, Yang C, Zhang J (2005) Research progress in fluid catalytic cracking technology. J Univ Pet China 29:135–150
Xin Q, Lin L (2013) Progress in catalysis in China during 1982–2012. Theory Technol Innov 34:401–435
Speight J (1999) The chemistry and technology of petroleum pp 395–435
Liu W, Liang Y, Liu Y, Yang J (2002) Effects of components on induction period of RFCC gasoline. Acta Petroleum Sinca (Petroleum Processing Section) 18:6–8
Liu Z, Liu J, Sun L (2001) Resid hydroconversion—a new approach in processing inferior residual oils. Acta Petroleum Sinica (Petroleum Processing Section) 17:35–41
Deldari H (2005) Suitable catalysts for hydroisomerization of long-chain normal paraffins. Appl Catal 293:1–10
Wilson S, Lok B, Messina C (1982) Aluminophosphate molecular sieves: a new class of microporous crystalline inorganic solids. J Am Chem Soc 104:1146–1147
Lok BM, Messina C, Patton R (1984) Silicoaluminophosphate molecular sieves: another new class of microporous crystalline inorganic solids. J Am Chem Soc 106:6092–6093
Xu B, Han X, Qian L, Wang H, Yan Z (2006) Application of CoAPO-11 molecular sieve for 1-hexene isomerization and its deactivation in the reaction. Chin J Catal 26:842–846
Xu B, Yan Z, Bai P, Zhao C, Han X, Huo Q (2004) Synthesis and characterization of MAPO-11 molecular sieves. J Univ Pet China 28:116–119
Wang Z, Yan Z (2001) Synthesis and characterization of Si- and Zr- substituted heteroatom-aluminophosphate zeolites. J Nat Gas Chem 10:158–167
Huang X, Wang L, Kong L, Li Q (2003) Improvement of catalytic properties of SAPO-11 molecular sieves synthesized in H2O-CTAB-butanol system. Appl Catal 253:461–467
Zhang S, Chen S, Dong P, Jia Z, Zhao J, Xu K (2007) Synthesis and catalytic hydroisomerization performance of SAPO-11 molecular sieves with small crystals. Chin J Catal 28:857–864
Ryoo R, Srivastava R, Choi M (2006) Organosilane surfactant-directed synthesis of mesoporous aluminophosphates constructed with crystalline microporous frameworks. Chem Commun 42:4380–4382
Fan Y, Xiao H, Shi G, Liu H, Bao X (2012) Alkylphosphonic acid- and small template synthesis of hierarchical silicoaluminophosphate molecular sieves with high isomerization selectivity to di-branched paraffins. J Catal 285:251–259
Lin X, Fan Y, Liu Z, Shi G, Liu H, Bao X (2007) A novel method for enhancing on-stream stability of fluid catalytic cracking (FCC) gasoline hydro-upgrading catalyst: post-treatment of HZSM-5 zeolite by combined steaming and citric acid leaching. Catal Today 125:185–191
Liu X, Yan Z (2001) Optimization of nanopores and acidity of USY zeolite by citric modification. Catal Today 68:14–154
Liu G, Jia M, Zhou Z, Wang L, Zhang W, Jiang D (2006) Synthesis and pore formation study of amorphous mesoporous aluminophosphates in the presence of citric acid. J Coll Inter Sci 302:278–286
Liu X, Yan Z (2000) Modification of USY zeolites with citric acid. Acta Chim Sinica 58:1009–1014
Liu Z, Song H, Feng Z, Yan Z (2013) Hierarchical meso-microporous SAPO-11 synthesis from acid assistant dealumination effect of acid strength. Appl Mech Mater 313–314:219–222
Cao G, Shah M, Brody J (2003) Synthesis of silicoaluminophosphates, US Patent 6927187, ExxonMobil Chemical Patents Inc
Acknowledgments
This work was supported by Science and Technology Development Project of CNPC (No. 11-13-01-05); National Science Foundation China (U1362202 and 21,206,196), Innovation Foundation of CNPC (2013D-5006-0404), and Funds for Distinguished Youngs of Shandong Province (BS2012NJ013).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Song, H., Liu, Z., Xing, W. et al. Synthesis of meso-SAPO-11 and its enhancement of isomerization in fluid catalytic cracking process. Appl Petrochem Res 4, 389–394 (2014). https://doi.org/10.1007/s13203-014-0078-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-014-0078-6