Abstract
In this study, bimetallic catalysts based on transition metals CuFe2O4, CoFe2O4 and NiFe2O4 are proposed for catalyzing aquathermolysis reaction during steam-based EOR method to improve in-situ heavy oil upgrading. All upgrading experiments were carried out under a nitrogen atmosphere for 24 h in a 300-ml batch Parr reactor at 250 and 300 °C under high pressure 35 and 75 bar, respectively. To evaluate the catalytic performance of the bimetallic catalysts used, comprehensive studies of changes in the physical and chemical properties of the improved oils, including the viscosity, elemental composition and SARA fractions of oils before and after upgrading processes were used. Furthermore, individual SARA fractions were characterized in detail by Gas Chromatography (GC), High-Performance Liquid Chromatography (HPLC) and Carbon-13 Nuclear Magnetic Resonance (13C NMR), respectively. The results showed that bimetallic catalysts have high catalytic performance at 300 °C for the upgrading of heavy crude oil in viscosity reduction, increasing the amount of saturates (especially alkanes with low carbon number) as a result of thermal decompositions of high molecular weight compounds like resin and asphaltenes leading to their increasing. Furthermore, the upgrading performance is reflected in the improvement of the H/C ratio, the removal of sulfur and nitrogen through desulfurization and denitrogenation procedures, and the reduction in polyaromatic content, etc. CoFe2O4 gives the best performance. Generally, it can be concluded that, used bimetallic based catalysts can be considered as promising and potential additives improving in-situ upgrading and thermal conversion the heavy oils.
Similar content being viewed by others
Introduction
Due to the depletion of light crude oil reserves around the world, heavy crude oil production will rise, implying that heavy oil resources have enormous potential to meet future demand for petroleum products (Zhang et al. 2012). On the other hand, the International Energy Agency (IEA) estimated that fossil fuels such as heavy crude oil, coal and natural gas would reach about 60% of global demand growth by 2030 (“Forecasting Supply and Demand up to 2030,” 2005). Heavy oil constitutes at least 25% of the world's oil reserves, a significant proportion of the estimated reserves of hydrocarbons. Huge deposits have proven to be associated with abundant light oil fields in a region like the Middle East. Moreover, there are thirty global countries listed with large heavy oil reserves, with the biggest reservoirs being the USA, Canada, and Venezuela (Hein 2006; Mokrys and Butler 1993). Heavy crude oil defined as unconventional crude oil, characterized by high viscosity (greater 1000 mPa.s), with an API gravity between 10 and 20 degrees, low hydrogen to carbon ratio, and a lot of contaminants (i.e., heteroatoms such as S and N and metals such as Ni and V). Heavy crude oil has a low flow rate, which makes transport quite challenging. Because of the high resin and asphaltene concentration, (both of them have a high average molecular weight) together with low content the saturated and aromatic hydrocarbons, heavy crude oil is often known for its poor quality (Iskandar et al. 2016). Based on these properties described above, the production of heavy oil can be a significant challenge, because it will not flow in a reservoir unless it's stimulated by one of the Enhanced Oil Recovery (EOR) methods. In addition, moving heavy oil by pipeline and refining it is time-consuming and costly. Therefore, the improvement of heavy oil inside reservoir is necessary to ensure ease of production and marketing, as this is achieved via in-situ upgrading techniques, using the reservoir as a free reactor (Hart and Wood 2018). In-situ upgrading can have benefits compared with the traditional surface upgrading technology such as enhances oil recovery, improves the performance of the well production, and reduces the expense of lifting from reservoir to the wellhead and transportation from oilfield to refinery and thus, it avoids the expense of building of catalytic reactors or vessels. The in-situ upgrading process can be implemented onshore or offshore and in remote regions, where surface services might be not available or unacceptable, as well as on a well-to-well basis, and therefore, it could be modified for lower production rates, while surface processing is designed for a specified volume range of the crude oil. Furthermore, it is considered as environmentally friendly (Mohammad and Mamora 2008). The aquathermolysis is an in-situ upgrading technique of heavy crude oil. The word “aquathermolysis” was used to describe the chemical interactions occurring between heavy oil, water, and reservoir matrix at steam-stimulation conditions (high temperature and pressure). The main cause of the aquathermolysis process can be clarified, as the chemical interactions, can break the C−S bond in heavy oil, reduce the amount of resin and asphaltene, and as a result, the viscosity of the heavy oil decreases (Clark and Hyne 1990; Liu 2002). Due to the reservoir temperature will gradually decrease after steam injection with the distance to the wellbore increases, it is hard to sustain over 240 °C, as a result, there is an inadequate supply of energy for aquathermolysis interactions. Therefore, it is necessary to use catalysts in the aquathermolysis reactions to improve the promotion technique, which can produce catalytic reaction under promotion conditions. The use of catalysts in the aquathermolysis process facilitates the cracking of C−S, C−O, and C−N bonds at low temperatures, thus reducing the heavy hydrocarbons content and increasing the light hydrocarbons, which leads to a reduction in viscosity (Ancheyta et al. 2010; Chen et al. 2008; Hamedi Shokrlu and Babadagli 2013; Maity et al. 2010; Suwaid et al. 2020). The effect of catalysts on aquathermolysis reactions in the steam injection process has been examined in several studies. Jacob B. Omajali et al. (2017) looked on in-situ catalytic upgrading of heavy oil with distributed nanoparticles supported by gram-positive and gram-negative bacteria. (Omajali et al. 2017). Many studies were carried out to evaluate the in situ catalytic upgrading of heavy oil using a pelletized Ni-Mo/Al2O3 catalyst and dispersed nanoparticulate iron oxide. In these studies, the optimal reaction temperature was from 325 to 425 °C (Al-Marshed et al. 2015; Hart et al. 2017; Hart and Wood 2018).
In this work, upgrading of heavy crude oil from oilfield in Tatarstan (Russia) using catalytic and non-catalytic aquathermolysis processes was studied. A series of experiments were conducted at different temperatures (250 and 300 °C) and three types of bimetallic oxide catalysts, (Copper Iron Oxide (CuFe2O4), (Cobalt Iron Oxide (CoFe2O4), and Nickel Iron Oxide (NiFe2O4)) to evaluate the effect of temperature reaction and type of catalyst on for improving the upgrading performance of heavy crude oil.
Experimental section
Materials
In this research, the heavy crude oil was taken from oilfield in Tatarstan (Russia). The studied heavy crude oil was taken from a shallow reservoir with a range depth of 50–250 m (Varfolomeev et al. 2016). The chemical and physical properties of the initial heavy crude oil are shown in Table 1.
Synthesis, preparation and characterization of bimetallic oxide catalysts
In distilled deionized water, a 0.4 M (25 ml) solution of iron (III) nitrate was mixed with 0.2 M (25 ml) solutions of cobalt, nickel, and copper nitrate. A 3M (25 ml) sodium hydroxide solution was prepared and dropped into the salt solution. The pH of the solution was checked on a regular basis as the sodium hydroxide solution was added. A magnetic stirrer was used to constantly stirred the reagents until a pH of 11–12 was obtained. As a surfactant and coating ingredient, oleic acid was dropped to the solution and heated to 80 °C for one hour (Pillai and Shah 1996). Then the product was cooled to ambient temperature. Distilled water and ethanol were used to remove excess surfactant from the solution. After that, the content of the beaker was centrifuged for 15 minutes at 3000 rpm to isolate the supernatant. After that, the supernatant was centrifuged until only a thick black precipitate remained. Then the precipitate was dried at 100 ° C for 12 hours. The substance was then crushed to a fine powder. At this stage, the product retains some bound water (up to 10% by weight), which was removed by heating it at 500°C for 10 hours. (Maaz et al. 2007).
In the chamber of the Quorum Q 150 T ES vacuum equipment, the samples are moved on the chuck. The conductive layer is created using a cathodic sputtering process and 80/20 Au / Pd alloy. The alloy is 15 nm thick. The catalysts were photographed using a Merlin Carl Zeiss high-resolution field emission scanning electron microscope. The Aztec X-Max energy-dispersive spectrometer is included in the scope of delivery of the microscope. To minimize the modification of the sample, a snapshot of the morphological surface was taken with an accelerating voltage of 15 kV and a current sensor of 300 pA.
Figure 1 shows the morphology of synthesis bimetallic oxide catalysts (CuFe2O3, CoFe2O3 and NiFe2O3). The results show that, the average size of the studied bimetallic particles was in the range of 0.8–1 µm.
Experimental apparatus and upgrading procedures
A series of suggested aquathermolysis experiments in the presence and absence of catalyst were carried out in a 300-ml batch Parr reactor (4575/76 HP /HT reactor) with a heating device and a temperature controller (Fig. 2). In non-catalytic aquathermolysis experiments, oil and distilled water were loaded into the reactor in a mass ratio of 70:30 g. In addition, in catalytic aquathermolysis experiments, batch reactor was loaded with oil, distilled water, and bimetallic oxide catalysts at a mass ratio of 70:30:0.42 g, respectively. In both reactions, to remove oxygen the reactor was merged with a nitrogen (inert medium). All of the experiments and conditions are illustrated in Table 2. Later, the oil samples were collected and separated for further analysis.

adapted from our previous work (Al-Muntaser et al., 2020))
External view of batch reactor (
Products separation and analytical procedures
Separation of oil/water emulsion
The available method of separating a mixture of oil and water is the centrifugation method. This process was carried out by using a centrifuge (Awel Model MF 20 / -R b 48 / -R) with a rotation speed of 5000 rpm at 40 °C for 2 h. The two components of the mixture were separated depending on their density, oil remains on the top and water will precipitate on the bottom of the centrifuge eppendorf.
Viscosity and elemental analysis measurements
The viscosity of the heavy crude oil samples before and after treatment was measured by a Brookfield Viscometer under at temperature of 25 °C. As part of the study, the elemental compositions including carbon, hydrogen, nitrogen and sulfur (CHNS) of initial heavy and improved oil samples were determined as a percentage of the mass using a Perkin Elmer–Elemental Analyzer 2400 Series II at a temperature of 1000 °C.
SARA fractions
Separation of oils, before and after upgrading, into four groups of chemical compounds (saturated hydrocarbons, aromatic compounds, resins, and asphaltenes (wt.%))- SARA analysis carried out according to ASTM D 4124 (Wang et al. 2019). However, the procedures for conducting SARA analysis are as follows:
-
Take 1 g of crude oil and put it in the flask and add 40 g of heptane as a precipitator of asphaltene (Fan and Buckley 2002). Agitate the mixture for 30 min and keep the mixture in a dark place for 24 h.
-
Filtrate the mixture using a filter paper (2.5 µm). Infiltrate component called maltane (saturated, aromatic, resin and heptane).
-
Take the filter paper and place it in the Soxhlet extractor device to remove the remaining maltane from asphaltene filtration paper that exists on filter paper at 110 °C and 120 rpm.
-
After that 180 ml of toluene used to extract asphaltene from filter paper by using Soxhlet extractor device at 100 °C and 120 rpm.
-
To separate toluene from asphaltene a rotary evaporator used at 40 °C, 77 mbar, and 130 rpm, then the asphaltene was weighed using a laboratory balance.
-
Maltane components separation is done by passing their mixture through chromatographic column (length 44 cm and internal diameter 20 cm) contained a small cotton in the end and aluminum oxide (Al2O3), the 2nd degree of activity by Brokmman of fraction 0.04–0.2 mm, as adsorbent that pr-calcined at 430 °C for 4 h.
-
Carefully pour 200 ml of heptane through the column to dissolve saturated hydrocarbons.
-
After saturated hydrocarbons dissolved by heptane, both of them infiltrate and collected in a flask.
-
Separate the solvent (heptane) from the mixture (heptane and saturate fraction) by distillation process using a rotary evaporator at 40 °C, 120 mbar, and 140 rpm. Then the remaining fraction (saturated hydrocarbons) is weighted.
-
Add 200 ml of toluene to the remaining maltane that exists in the column to dissolve aromatic hydrocarbons and collected in another flask.
-
Separate the solvent (toluene) from the mixture (toluene and aromatic hydrocarbons) by distillation process using a rotary evaporator at 40 °C, 77 mbar, and 140 rpm. Then the remaining fraction (aromatic hydrocarbons) is weighted.
-
Add a mixture (100:100 ml of toluene and isopropyl alcohol) to the remaining maltenes that exist in column to dissolve the resin and collected in another flask.
-
Separate the solvent (toluene and isopropyl alcohol) from the mixture (toluene, isopropyl alcohol and resin fraction) by distillation process using a rotary evaporator at 40 °C, 144–77 mbar, and 140 rpm. Then the remaining fraction (resin) is weighted. The SARA separation scheme is shown in Fig. 3.
The illustration scheme of the SARA separation process (adapted from (Al-Muntaser et al., 2020))
Gas chromatography analysis
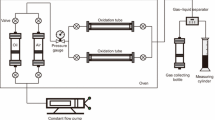
The study of saturated hydrocarbons by gas chromatography before and after upgrading was investigated. The disruption of n-alkanes in saturated hydrocarbons of heavy crude oil before and after upgrading was investigated using an Agilent 7890A gas chromatography. The description of the analysis methodology is described in detail in our previous work (Djimasbe et al. 2020).
HPLC analysis
A Dionex Ultimate 3000 HPLC system (Dionex, Germany) was used to determine the relative amounts of methane-nafthene, mono-aromatic, di-aromatic, and tri-aromatic in aromatic hydrocarbon fractions. The usual HPLC operating pressure was around 105 bar, and the column temperature was sustained at 35 °C using a column heater module. For instrument control and chromatographic data collecting, the Dionex ChromelonTM Chromatography Data System was used.
NMR analysis
NMR experiments on studied samples of resin fractions dissolved in CDCl3 were performed on a Bruker AVANCE-III-HD-500 NMR spectrometer. The deuterium signal from the CDCl3 solvent was used to establish field lock and shimming. In our prior work (Rakhmatullin et al. 2018; Suwaid et al. 2020), we detailed the parameters and processes for acquiring and analyzing the 13C NMR spectra. Figure 4 shows the principal scheme of catalytic and non-catalytic upgrading and the analysis procedure of obtained products.
Results and discussion
Viscosity of oil samples before and after upgrading
The results of the viscosity of the crude oil before and after upgrading processes by catalytic and non-catalytic aquathermolysis reactions at 250 and 300 °C, respectively, for 24 h are shown in Fig. 5. The results showed that the viscosity at 25 °C was decreased from 2073 mPa.s (initial oil) to 1758 mPa.s after treated in absence of catalysts (non-catalytic aquathermolysis reactions) at 250, and at 300 °C a slight increase in viscosity was observed from 2073 mPa.s (initial oil) to 2336 mPa.s. The increasing of viscosity at 300 °C compared with 250 °C was due to increasing the content of asphaltene and resin and this may indicate that the condensation reactions had begun to prevail. The main mechanism for the formation of coke is through the condensation or recombination reactions of free radicals, which are also one of the main reasons for an increase in improved oil viscosity. With catalytic aquathermolysis reactions, decreasing crude oil viscosity was observed under the same conditions with non-catalytic aquathermolysis reactions alone, as explained in experimental section (Table 2). Acceding to the results of Fig. 5, it is easy to see that the other conditions remain unchanged, the heavy oil viscosity reduction rate increased gradually and reached to 12, 18, and 33% at 250 °C and 50, 54 and 49% at 300 °C, respectively, with catalysts (NiFe2O4, CoFe2O4, and CuFe2O4). The catalyst could improve the conversion quality of the heavy oil. Generally, the heavy oil had been upgraded to light oil and this showed how much the upgrading is an effective process.
Elemental analysis, desulfurization and denitrogenation of oil samples during thermal upgrading process
Table 3 shows the results of elemental composition of oil before and after upgrading. The elemental composition of the oil was changed after upgrading processes, the carbon content in oil begun lower, hydrogen content increased, (H/C) atomic number ratio is higher, and the ratio of heteroatom containing to carbon is lower. The higher (H/C) atomic number ratio is an indicator of improved quality of the heavy oil because the content of sulfur is reduced in with more light hydrogen components. Because the desulfurization and denitrogenation processes the elemental content of sulfur and nitrogen was decreased. The desulfurization and denitrogenation process occurs during upgrading reaction with catalysts due to the presence of Fe3+, Co2+ and Ni2+ ions in catalysts that catalyze the cleavage of the C−S and C−N bonds compare to hydrothermal cracking without catalysts (Li et al. 2019). The bonds energy of C−S and C−N are 272 and 305 kJ/mole, respectively, weaker than C−C bond which have 346 kJ/mole, that able to cleavage C−S and C−N bonds more easily (Anugwom et al. 2011; Dharaskar et al. 2014; Tang et al. 2017).
Changes in SARA fractions
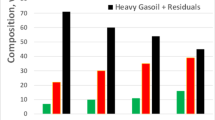
SARA analysis of crude oil before and after treatment in the presence and absence of catalysts at 250 and 300 °C for 24 h is shown in Fig. 6. After treatment, aquathermolysis, in the absence of catalysts at 250 and 300 °C, the content of saturated hydrocarbons were slightly increased, while the content of aromatic compounds, resins, and asphaltenes was slightly decreased at 250 °C but the content of these compounds was increased after treatment at 300 °C compared with the initial oil. After that, an increase in viscosity was noted as a result of this process. After an upgrade by using catalytic aquathermolysis reactions at 300 °C in the presence of catalysts (CoFe2O4), (CuFe2O4), and (NiFe2O4), a significant increase in the content of saturated hydrocarbons, especially with CuFe2O4 and NiFe2O4. A slight decrease in resins and asphaltenes content was observed. Generally, the use of catalysts helps in the destruction of resins and asphaltenes which had high molecular weight and then convert into low molecular weight.
Gas-chromatography analysis of saturated hydrocarbons before and after upgrading processes
Figures 7, 8 show gas chromatographic analysis and chromatogram of the saturated hydrocarbons of initial oil, after aquathermolysis upgrading, and catalytic aquathermolysis upgrading processes at temperatures 250 and 300 °C. According to the results in figures, upgrading of oil with non-catalytic aquathermolysis reactions caused little changes in alkanes with light molecule weight in the saturate (C9–C20). After upgrading in the presence of catalysts, the content of low-molecular-weight alkanes was clearly increased. By raising the temperature and pressure, some long chains of heavy hydrocarbon components were broken down to light alkanes by pyrolysis and ring opening of resin and asphaltene molecules.
High performance liquid chromatography (HPLC) analysis of aromatic hydrocarbons before and after upgrading processes
The results of high performance liquid chromatography analysis of the aromatic hydrocarbons for oil samples before and after upgrading by catalytic and non-catalytic aquathermolysis processes at temperatures 250 and 300 °C are shown in Table 4. According to the results in Table 4, a decrease in aromatic hydrocarbons is observed compared with the aromatic hydrocarbon of initial oil. However, the intensity of mono-aromatic hydrocarbons increasing, especially after non-catalytic aquathermolysis at 300 °C. A decrease in di-aromatic hydrocarbons is observing, especially after catalytic aquathermolysis upgrading with CoFe2O4 at 300 °C. These changes probably due to the decomposition of tri-aromatic hydrocarbons. For methane-nafthene, the device is not detected (ND).
Nuclear magnetic resonance (NMR) spectroscopy analysis of oil before and after upgrading processes
The resins before and after upgrading processes in the absence and the presence of catalysts at 250 and 300 °C were studied by 13C NMR spectroscopy as shown in Fig. 9 and Table 5. In the high (right spectral region) and low (left spectral region) fields, aliphatic and aromatic proton signals have distinct spectral regions. 13C NMR spectra contain a plethora of distinct signals, that can be allocated to various typical regions and so provide information on the Molar fractions of primary (methyl groups CH3)–Cp, secondary ((methylene groups CH2) + quaternary (C))–Csq, tertiary (methane groups CH)–Ct, aromatic (Car) groups, aromaticity factor (FCA), and mean chain length (MCL) of aliphatic hydrocarbons.
The content of Cp and Ct hydrocarbon groups grows with simultaneous exposure to high temperature and catalysts according to the data in Table 5. At the same time for Csq and Car hydrocarbon groups, there is a reverse process. More detailed analysis showed that the strongest effect on increasing concentration of Cp parameter exerts by CuFe2O4 at temperature of 300 °C. Approximately double increase was observed in the concentration of Ct used aquathermolysis reaction at 300 °C and catalytic aquathermolysis reactions with CuFe2O4 and CoFe2O4 at 250 °C. Also, the maximum increase was noted with CuFe2O4 at 250 °C and NiFe2O4 at 300 °C. For the Csq parameter, it was observed that aquathermolysis reactions had a small effect on these hydrocarbon groups (a slight increase at 300 C° from 38.8 to 39.5%). The effect of various catalysts (CuFe2O4, CoFe2O4, and NiFe2O4) at 250 and 300 °C showed a slight increase in the Csq hydrocarbon group share and the maximum value 44% was observed at 300 °C with NiFe2O4. The share of aromatic hydrocarbon groups Car and aromaticity factor (FCA) declined to the minimum values when aquathermolysis reaction at 300 °C. Although, in this case the catalysts also lead to a decrease in the Car hydrocarbon groups; however, their influence is not strong enough than with a simple temperature effect. Also was noted that the mean chain length (MCL) slightly lengthen after aquathermolysis and catalytic aquathermolysis reactions at 250 and 300 °C. However, as an exception, exposure by CuFe2O4 at 250 °C, CoFe2O4 and NiFe2O4 at 300 °C break the general trend. This is probably due to the unusual effect of these two conditions on the concentration of Ct hydrocarbon groups.
Conclusions
In this work, bimetallic catalysts based on transition metals (CuFe2O4), (CoFe2O4) and (NiFe2O4) are proposed for catalyzing aquathermolysis reaction during steam stimulation to improve in-situ heavy oil upgrading. For non-catalytic aquathermolysis at 250 °C showed a slight improving in the upgrading, where viscosity decreased, with an increase in the saturated and aromatic hydrocarbons content, in addition to that, a decrease in the content of resin and asphaltene. In catalytic aquathermolysis processes, catalysts (CuFe2O4, CoFe2O4, and NiFe2O4) had an effective role, when using them at 250 °C, they showed noticeable close results for the upgrading process. On other hands, the use of catalytic aquathermolysis processes at 300 °C was the best process used in this study, as it showed clear superiority and high efficiency in the upgrading process, especially when using NiFe2O4, as its viscosity decreased to the lowest value compared with other results. Furthermore, according to GC analysis after catalytic aquathermolysis processes, that some long chains of the heavy hydrocarbon components were broken down to light alkanes due to pyrolysis and ring-opening of resin and asphaltene molecules when increasing the temperature and pressure. Based on the obtained results, it can be concluded that the used catalysts were the best selection as a promised materials for conversion the heavy oil to light. Finally, from the above, we can conclude that the in-situ upgrading of heavy oil using catalytic and non-catalytic aquathermolysis technique is a promising technique for enhanced oil recovery with efficient features such as producing high-quality crude oil, reducing production and transportation costs by reducing energy consumed, and it’s considered as environmentally friendly.
References
Al-Marshed A, Hart A, Leeke G, Greaves M, Wood J (2015) Optimization of Heavy Oil Upgrading Using Dispersed Nanoparticulate Iron Oxide as a Catalyst. Energy Fuels 29:6306–6316. https://doi.org/10.1021/acs.energyfuels.5b01451
Al-Muntaser AA, Varfolomeev MA, Suwaid MA, Yuan C, Chemodanov AE, Feoktistov DA, Rakhmatullin IZ, Abbas M, Domínguez-Álvarez E, Akhmadiyarov AA (2020) Hydrothermal upgrading of heavy oil in the presence of water at sub-critical, near-critical and supercritical conditions. J Pet Sci Eng 184:106592
Ancheyta J, Trejo F, Rana MS (2010) Asphaltenes: chemical transformation during hydroprocessing of heavy oils. CRC Press
Anugwom I, Mäki-Arvela P, Salmi T, Mikkola J-P (2011) Ionic liquid assisted extraction of nitrogen and sulphur-containing air pollutants from model oil and regeneration of the spent ionic liquid. J Environ Prot 2:796
Chen Y, Wang Y, Wu C, Xia F (2008) Laboratory experiments and field tests of an amphiphilic metallic chelate for catalytic aquathermolysis of heavy oil. Energy Fuels 22:1502–1508
Clark PD, Hyne JB (1990) Studies on the chemical reactions of heavy oils under steam stimulation condition. Aostra J Res 29:29–39
Dharaskar SA, Wasewar KL, Varma MN, Shende DZ, Tadi KK, Yoo CK (2014) Synthesis, characterization, and application of novel trihexyl tetradecyl phosphonium bis (2, 4, 4-trimethylpentyl) phosphinate for extractive desulfurization of liquid fuel. Fuel Process Technol 123:1–10
Djimasbe R, Varfolomeev MA, Al-Muntaser AA, Yuan C, Suwaid MA, Feoktistov DA, Rakhmatullin IZ, Milovankin AA, Murzakhanov F, Morozov V (2020) Deep Insights into Heavy Oil Upgrading Using Supercritical Water by a Comprehensive Analysis of GC, GC–MS, NMR, and SEM–EDX with the Aid of EPR as a Complementary Technical Analysis. ACS Omega 6:135–147
Fan T, Buckley JS (2002) Rapid and accurate SARA analysis of medium gravity crude oils. Energy Fuels 16:1571–1575. https://doi.org/10.1021/ef0201228
Hamedi Shokrlu Y, Babadagli T (2013) In-situ upgrading of heavy oil/bitumen during steam injection by use of metal nanoparticles: A study on in-situ catalysis and catalyst transportation. SPE Reserv Eval Eng 16:333–344
Hart A, Wood J (2018) In situ catalytic upgrading of heavy crude with CAPRI: influence of hydrogen on catalyst pore plugging and deactivation due to coke. Energies 11:636
Hart A, Wood J, Greaves M (2017) In situ catalytic upgrading of heavy oil using a pelletized Ni-Mo/Al2O3 catalyst in the THAI process. J Pet Sci Eng 156:958–965
Hein FJ (2006) Heavy oil and oil (tar) sands in North America: an overview & summary of contributions. Nat Resour Res 15:67–84
Iskandar F, Dwinanto E, Abdullah M, Muraza O (2016) Viscosity reduction of heavy oil using nanocatalyst in aquathermolysis reaction. KONA Powder Part J 33:3–16
Li C, Huang W, Zhou C, Chen Y (2019) Advances on the transition-metal based catalysts for aquathermolysis upgrading of heavy crude oil. Fuel 257:115779
Liu Y (2002) Study on the aquathermolysis and viscosity reduced mechanism of heavy oil. JOURNAL-DAQING Pet Inst 26:95–98
Maaz K, Mumtaz A, Hasanain SK, Ceylan A (2007) Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J Magn Magn Mater 308(2):289–295. https://doi.org/10.1016/j.jmmm.2006.06.003
Maity SK, Ancheyta J, Marroquín G (2010) Catalytic aquathermolysis used for viscosity reduction of heavy crude oils: a review. Energy Fuels 24:2809–2816
Mohammad, A.A.A., Mamora, D.D., 2008. Insitu upgrading of heavy oil under steam injection with tetralin and catalyst, in: International Thermal Operations and Heavy Oil Symposium. Society of Petroleum Engineers.
Mokrys, I.J., Butler, R.M., 1993. In-situ upgrading of heavy oils and bitumen by propane deasphalting: the VAPEX process, in: SPE Production Operations Symposium. Society of Petroleum Engineers.
Omajali JB, Hart A, Walker M, Wood J, Macaskie LE (2017) In-situ catalytic upgrading of heavy oil using dispersed bionanoparticles supported on gram-positive and gram-negative bacteria. Appl Catal B Environ 203:807–819
Pillai V, Shah DO (1996) Synthesis of high-coercivity cobalt ferrite particles using water-in-oil microemulsions. J Magn Magn Mater 163(1–2):243–248. https://doi.org/10.1016/S0304-8853(96)00280-6
Rakhmatullin IZ, Efimov SV, Tyurin VA, Al-Muntaser AA, Klimovitskii AE, Varfolomeev MA, Klochkov VV (2018) Application of high resolution NMR (1 H and 13 C) and FTIR spectroscopy for characterization of light and heavy crude oils. J Pet Sci Eng 168:256–262
Forecasting Supply and Demand up to 2030, 2005. . Int. Energy Agency.
Suwaid MA, Varfolomeev MA, Al-Muntaser AA, Yuan C, Starshinova VL, Zinnatullin A, Vagizov FG, Rakhmatullin IZ, Emelianov DA, Chemodanov AE (2020) In-situ catalytic upgrading of heavy oil using oil-soluble transition metal-based catalysts. Fuel 281:118753
Tang XD, Chen XD, Li JJ, Deng LY, Liang GJ (2017) Experimental study on homogeneous catalytic upgrading of heavy oil. Pet Chem 57:1018–1023
Varfolomeev MA, Galukhin A, Nurgaliev DK, Kok MV (2016) Thermal decomposition of Tatarstan Ashal’cha heavy crude oil and its SARA fractions. Fuel. https://doi.org/10.1016/j.fuel.2016.08.042
Wang J, Wang T, Hou X, Xiao F (2019) Modelling of rheological and chemical properties of asphalt binder considering SARA fraction. Fuel 238:320–330
Zhang, Z., Barrufet, M.A., Lane, R.H., Mamora, D.D., 2012. Experimental study of in-situ upgrading for heavy oil using hydrogen donors and catalyst under steam injection condition, in: SPE Heavy Oil Conference Canada. Society of Petroleum Engineers.
Acknowledgements
This work was supported by the Ministry of Science and Higher Education of the Russian Federation under Agreement No. 075-15-2020-931 within the framework of the development program for a world-class Research Center “Efficient development of the global liquid hydrocarbon reserves.”
Funding
No particular funding for this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
AL-Rubaye, A.H., Suwaid, M.A., Al-Muntaser, A.A. et al. Intensification of the steam stimulation process using bimetallic oxide catalysts of MFe2O4 (M = Cu, Co, Ni) for in-situ upgrading and recovery of heavy oil. J Petrol Explor Prod Technol 12, 577–587 (2022). https://doi.org/10.1007/s13202-021-01311-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-021-01311-1