Abstract
Corrosion is considered one of the major problems that affect flow assurance during hydrocarbon production. This irreversible phenomenon has the ability to cause serious material failure in the oil and gas industry. Consequently, heavy capital and operational costs are required to prevent corrosion processes. Sweet corrosion of raw gas production facilities in an Algerian gas field manifests inside surface installations, which leads to gas production interruption and high intervention costs. To mitigate this type of corrosion, many methods can be applied such as the selection of appropriate materials, the injection of inhibitors, and the use of protective coating. In this work, the main points of gas production system that have been affected by corrosion and the inspection techniques used in the studied field were reviewed. Moreover, the efficiency of two types of two corrosion inhibitors (film-forming/neutralizing and film-forming chemicals) was studied by measuring the corrosion rate in the field and conducting chemical analyses on the produced water samples in the laboratory. The results of laboratory analyses regarding pH and iron content measurement point out that the injection of film-forming/neutralizing chemical significantly shifted the pH of the medium from acid to more neutral value and decreased the iron content, while the injection of film-forming inhibitor affected only the iron content by decreasing its tenor in the water samples. These results confirm the functions of each inhibitor to protect metal against internal corrosion. The comparison between the single- and double-function inhibitors reveals that the film-forming inhibitor (CK981DZ) outperforms the film-forming/ neutralizing chemical with an efficiency that exceeds 99% at low injection rate. Moreover, it provides good compatibility and stability all through its injection path. Meanwhile, the application of double function inhibitor (film-forming/neutralizing) significantly reduced the corrosion rate of carbon steel structures, but it involved the formation of deposits in the gas processing plant. The findings from this study can help give a better understanding of the methodology of corrosion inhibitor performance evaluation in real condition of gas production, also the criteria of inhibitor screening based on laboratory and field tests.
Similar content being viewed by others
Introduction
Corrosion is the most widespread degradation phenomenon in the oil and gas industries. It is one of the flow assurance issues that can arise anywhere along hydrocarbons paths including, but not limited to, production tubing, downhole valves, wellhead, surface facilities, pipelines, and processing plants (Kermani and Smith 1997; Popoola et al. 2013). Therefore, it is the origin of the majority of pressure equipment failure. Economically, It has been estimated that corrosion destroys a quarter of the world’s annual production in steel, which represents approximately 150 million tons per year, or 5 tons per second (Davis 2000). Tems et al. (Tems and Al-Zahrani 2006) reported that the economic costs associated with the corrosion of natural gas processing plants and oil refining plants range between 10 and 30% of the maintenance budget.
Carbon steel is widely used in petroleum and industries, basically due to its low capital expenditure cost (Hill et al. 2011; Mesquita and Marchebois 2020). The main problem of using carbon steel is its dissolution in acidic solutions (Aiad et al. 2014), in the other hand, the most common types of corrosion occurrences in these industries are general and localized corrosion (Finšgar and Jackson 2014). The other large problem in operating pipe flow lines is internal corrosion (Ghareba and Omanovic 2010; Askari et al. 2019), mainly due to stress corrosion cracking. Martinez et al. (Videm and Koren 1993) claim that the combination of corrosion and erosion is the main problem in pipe deterioration.
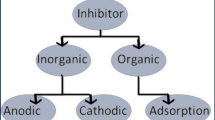
Several methods are reviewed in the literature to prevent the internal corrosion in oil and gas industries including the selection of appropriate methods, the application of inhibitors, and the use of internal protective coatings (Popoola et al. 2013; Papavinasam 2013; Ali et al. 2020).
Chemical prevention through the injection of inhibitors is a more cost-effective method in protecting facilities against internal corrosion (Garcia-Arriaga et al. 2010; Trabanelli 1991; Park et al. 2009; Stewart et al. 2009; Davoudi et al. 2014; Obot et al. 2020). Therefore, a range of chemicals have been reported in the literature to control this type of corrosion (Finšgar and Jackson 2014; Miksic et al. 2009; Javidi et al. 2019). However, the selection of an appropriate chemical formulation is still a big challenge due to the complexity of the corrosion sources and the diversity of the fluid compositions in a single field. Hence, the composition of the inhibitor in one well might not be effective in other wells from the same field. It is worth mentioning that the choice of an inhibitor must take into consideration: the materials to be protected, velocity and fluid flow regime, and the nature of the effluents (Finšgar and Jackson 2014; Miksic et al. 2009).
For the prevention of CO2 and H2S corrosions, which are the most prevalent attacks known in the oil and gas industries (Kermani and Morshed 2003; T. das Chagas Almeida, M.C.E. Bandeira, R.M. Moreira, O.R. Mattos 2017), the film-forming inhibitors are particularly useful. These types of inhibitors can be applied in continuous injection or batch treatment either downhole or at the wellhead (Glasgow et al. 2013), where their typical dosages are often in the range of 10 to 100 ppm. Many methods have been reported recently to optimize the dosage of film-forming inhibitors (Miksic et al. 2009; Mackenzie et al. 2010; Jing et al. 2018).
The effectiveness of a film-forming inhibitor is partly determined by the strength of its adsorption on the metal surface and forming a protective layer that prevents corrosive aqueous fluids from penetrating the metal surface (Ramachandran and Bartrip 2003; Wong and Park 2009; Askari et al. 2018).
In the case when no or little formation water is transported with hydrocarbons, a pH stabilizer can be used to protect gas pipelines against corrosion. This product helps in adjusting the pH of the medium which reduces the solubility of iron carbonate. This increases the deposition rate of iron carbonate and leads to the formation of a protective film, which consequently decreases the corrosion rate (Nyborg 2009).
This problem of corrosion manifests inside production surface facilities of raw gas in the studied Algerian field, like piercing and bursting of pipes leading to production interruptions and higher maintenance costs. This paper reviews the main points of the production system affected by corrosion and the program adopted to survey the unstable phenomenon of carbon steel corrosion. Moreover, the present work investigates the efficiency of two categories of inhibitors through the measurement of corrosion parameters and corrosion rate. Thus, two amine-based commercial inhibitors were studied. The first chemical is a film-forming /neutralizing inhibitor, which provides a neutralizing function of carbonic acid by the raising of the pH of the environment, and the covering function to isolate the aggressive water from the metal surface. The second chemical is a film-forming inhibitor, which offers only a covering function of the carbon steel structure. To our knowledge, this is the first study of this type that has been reported in the literature. This work differs from previous studies in the following points: the review of the gas production facilities damaged by internal sweet corrosion and the monitoring protocol to assess this type of corrosion, the assessment of corrosion inhibitor performance in real conditions of the gas field, and the comparison between two different chemicals to protect metals against corrosion are reported for the first time.
Generally, the origin of the corrosion in this field is complex, however, it is mainly promoted by the presence of carbon dioxide in crude gas composition. The key species that are chemically reduced in CO2 corrosion are H+, HCO3−, and H2CO3, which are all present in the water phase (Dayalan et al. 1998). After corrosion has been initiated, the iron will be oxidized to iron ions and with carbonate ions may produce (CO3−−) scales of FeCO3 (Kelland 2014) according to the corrosion mechanism presented in Fig. 1.
In the studied gas field, corrosion has a negligible effect on the production tubing, wellhead, descent pipe, and the first pipe upstream of the chock, because they are made of stainless steel. The rest of the facilities are carbon steel structures, and the type of the affected corrosion is localized inside the pipes (Fig. 2), which is promoted by CO2 (Martínez et al. 2009). This phenomenon is accentuated by the mechanical effect of erosion because of the gas flow velocity which can reach 11 m/s. The following facilities are exposed to this kind of corrosion:
-
Well surface equipment: carbon steel pipes downstream of the choke are subjected to severe hydrodynamic regimes due to the geometric variation of the pipe,
-
Junctions, manifolds gas producing wells, and the different collectors,
-
Corrosion occurs mainly at the welding cord and particularly at the lower generating part of the pipes; because every two pipes are joined by welding a flange, and the low points of the collectors constitute a favourable site stagnation of aggressive aqueous fluids where the erosion phenomenon reaches its maximum.
The external coating from painting these surface production facilities and the dry climate in this region help in avoiding the formation of external corrosion.
Corrosion has many causes, and its effects are different (Popoola et al. 2013; Papavinasam 2013). Therefore, there is no method that provides all of the answers. However, a combination of several methods allows for better identification of problems and solutions. Hence, a periodic monitoring should be carried very carefully to avoid failures and thereby ensure a long life for the equipment. The control program established in this gas field regroups the non-destructive method and chemical laboratory analyses.
Visual inspection is carried out to observe possible corrosion on the metal surface (Fig. 3). Using the naked eye discloses only the major flaws such as excessive deformation (definitive loss of the original geometry of the equipment). Thus, a better and non-destructive method is required to detect defects inside the material. This can be done by using an ultrasound instrument, based on transmission and reflection of ultrasonic waves inside the piece to be tested through a sensor, in which the frequencies are higher than the frequencies audible by the human ear. For this type of test, frequencies between 100 kHz and 25 MHz are required.
The ultrasound sensor is placed on four points upstream and downstream of the welding cord (Fig. 4) and it emits ultrasonic waves, which propagate inside the material at various speeds depending on the environment itself and the type of waves used. At the end of the measurement, a direct value of the thickness appears on the apparatus screen. This value is then compared to a threshold called the minimum calculus thickness to assess the state of the piece to be tested.
Beside the non-destructive technique, laboratory analyses of corrosion parameters such as pH and iron content in the water sample (produced water) are systematically conducted to assess the evolution of corrosion and to optimize the injection rates of corrosion inhibitor. However, this monitoring protocol can be adopted for any gas field.
In this work, the performance of two different commercial corrosion inhibitors is evaluated in an Algerian gas field based on field and laboratory tests and analyses. Thus, the rest of this paper is organized as follows. First, the experimental procedure is described in detail. Then, the results are reported and discussed. Finally, the main findings of the study are summarized in the last section.
Experimental procedure
The evaluation of the effectiveness of two commercial inhibitors was studied on two gas producer wells (A and B). One of the chemicals is film-forming/neutralizing and the second inhibitor is film-forming so-called CK981DZ which is purchased from Champion. The selected inhibitors are amine-based, whereas some of their physical properties are illustrated in Table 1. The presence of N–H amine functional groups in both chemicals has been recorded (Fig. 5) by infrared spectroscopy analysis using FTIR NICOLET iS50 spectrometer.
The film-forming/neutralizing inhibitor was injected in well A, meanwhile, the film-forming chemical was used in the well B. The chemical composition of the produced gas from the selected wells is presented in Table 2.
Each inhibitor was injected in the first pipe (testing pipe) downstream of the choke using a dosing pump as shown in Fig. 6. The pump is powered by solar panels and calibrated to provide the optimum concentration of the inhibitor. However, the continuous treatment is carried out in two stages: The first is to make a shock treatment required for forming a layer of the inhibitor on the testing pipe surface (the period of this step reached 24 h with an injection rate of 20 g/m2/d), and the second step is to maintain the inhibitor film; the concentration of the inhibitor is reduced to a low dose in this mode of treatment in order to optimize its injection rate, and the operator must ensure the proper functioning of the pump and the continuous availability of the corrosion inhibitor.
The procedure implemented in this study to assess the performance of corrosion inhibitors involves the laboratory analyses of the formation water and the measurement of corrosion rate before and after inhibitor injection.
During water sampling, we endeavoured to take only water and purge the associated gas. To investigate the performance of the injected inhibitor, all along the produced gas flow path, water samples were taken from four points as illustrated in Fig. 7.
The measurement of water pH is the key factor to optimize and adjust the injection rates; we set as a target, a pH value between 5.9 and 6.2 for better medium neutralization to avoid carbonate scale deposition. The iron content was also determined by titration based on the oxidation of ferrous iron to ferric iron in solution, which gives the mass concentration of iron in the solution. In our case, ethylene diamine tetra-acetic acid (EDTA) was used to determine iron content: 0.5 ml of concentrated HNO3 was added to 50 ml of water sample and shaken very well. The mixture was heated to boiling then cooled. In this stage, five drops of sulphosalicylic acid (1 wt%) was injected into the resulting mixture. The pH of this solution was adjusted by NaOH (2 N) to stabilize the iron complex (pH between 2.22 and 2.26). After this, the solution was titrated with EDTA (0.004 g/ml) to turn the colour from purple to light yellow and to calculate in the end the iron content using Eq. (1):
The measurement of corrosion rate before and after inhibitor injection provides us a general idea of inhibitor effectiveness. This measurement is carried out using an electric instrument placed into the pipe, where its low part is made of carbon steel and dissolves more quickly following the corrosive medium, and the value of corrosion rate can be directly read on the apparatus screen; then, this value is used to calculate the inhibitor efficiency "η" by applying the following equation:
where Vcor b and Vcor a are the corrosion rate measured before and after inhibitor injection, respectively.
Results and discussions
The results of pH analysis in four points before and after film-forming/neutralizing inhibitor injection into the first pipe downstream the shock of the well (Fig. 8) show an increase in the pH value in the testing pipe from 5.96 to 6.49 after inhibitor injection. Then, this value decreased because of a problem in the injection pump causing a lower injection rate. The evolution of pH in the junction inlet increased after the injection from 5.42 to 6.30. The same observation was noticed for the pH of the medium after inhibitor injection in the collector departure and processing plant inlet from 5.26 to 6.45 and from 5.66 to 6.48, respectively. This increase in pH value confirms the neutralizing action of film-forming/neutralized chemical.
According to Fig. 9, a net decrease in iron content is observed in four points after film-forming/neutralizing inhibitor injection, which means that the film formed on the pipe by this inhibitor acts as a barrier that separates the metal surface from the corrosive medium.
After the injection of film-forming inhibitor into the first pipe downstream the shock of well 2, there is no significant change in pH of the medium (Fig. 10), where its value is relatively constant with an average of 4.8 to 5.5. Nevertheless, a net reduction in the iron content is observed in the presence of the inhibitor in all points:
from 250 to 35 ppm in the testing pipe, from 210 to 40 ppm in the junction inlet, from 180 to 80 ppm in the collector departure, and from 180 to 60 ppm in the gas processing plant inlet. This means that the injection of this inhibitor influences only the iron content by forming a protective film on the metal surface against the carbonic acid.
From the corrosion rate measurements without and with inhibitor injection, it was possible to calculate the efficiency of each inhibitor:
The results of corrosion rate measurement before and after inhibitor injection show that both inhibitors reduced the corrosion rate. Moreover, the film-forming chemical provided the best protection of the pipeline against corrosion. The unknown composition (the chemical structures) of these commercial inhibitors makes the prediction of the inhibition mechanism more difficult.
In order to optimize the injection rate of corrosion inhibitor, both inhibitors were injected at different rates and the content of iron was measured for each injection rate (Fig. 11). However, the evolution of iron content as a function of the inhibitor injection rate shows that the optimum injection rate for a minimum iron content is about 1.02 l/million sm3 for film-forming/neutralizing inhibitor (Fig. 11a), and 0.5 l/ million sm3 for film-forming inhibitor (Fig. 11b).
It is worth noting that the selection of a good inhibitor is not limited by the efficiency of the chemical in protecting carbon steel object, but it covers also its stability and compatibility with the environment (Rahuma 2014; Keewan et al. 2018). Otherwise, it can promote other issues such as the formation of foam, emulsion, and deposits. In this work, the film-forming/neutralizing inhibitor provides a good performance against corrosion. Nevertheless, its injection caused the formation of deposits at the processing plants. In contrast, the film-forming chemical shows great protection with good stabilization (Table 3). It is important to note that it was impossible to detect at which concentration (so-called critical point) the film-forming/neutralizing inhibitor had started the formation of deposits in the processing plant.
Summary and conclusions
The following conclusions can be drawn from this study:
-
Corrosion of raw gas production surface facilities in our studied Algerian gas field occurs inside the carbon steel structures by the chemical attack of H2CO3, which causes perforations leading to leaks of effluent, and consequently to disturbances in the production of raw gas in this field.
-
Detection of the corrosion problem in this field is done through the application of specific inspection techniques represented essentially in the chemical laboratory analyses and non-destructive test by piping thicknesses measurement using an ultrasound apparatus.
-
The impact of corrosion inhibitors was analyzed in this study by the measurement of corrosion rate in the field and the chemical examination of pH and iron content of the associated water in laboratory before and after the injection of a film-forming and film-forming/neutralizing commercial chemicals. However, the results of this study show that both amine-based inhibitors increased the resistance of the carbon steel structure against corrosion.
-
Regrouping more than one function; film-forming and neutralizing actions, in a chemical may increase its capacity to protect carbon steel in the presence of the formation water. Nevertheless, this hypothesis is not always true according to the obtained results. However, the film-forming inhibitor (CK981DZ) provides the best protection of carbon steels comparing to the film-forming/neutralizing chemical.
-
The stability and compatibility of corrosion inhibitor with the produced fluids and the other injected chemicals were taken into consideration during the choice of inhibitors. Consequently, the formation of deposit was observed at the processing plant after the injection of the film-forming/neutralizing inhibitor. This outcome reduces the chance of selecting this inhibitor, despite its good performance to protect carbon steel structures.
Abbreviations
- η:
-
Inhibitor efficiency (%)
- Vcor:
-
Corrosion rate (μm/year)
References
Aiad IA, Tawfik SM, Shaban SM, Abd-Elaal AA, El-Shafie M (2014) Enhancing of corrosion inhibition and the biocidal effect of phosphonium surfactant compounds for oil field equipment. J Surfactants Deterg 17:391–401
Ali MM, Magee JC, Hsieh PY (2020) Corrosion protection of steel pipelines with metal-polymer composite barrier liners. J Nat Gas Sci Eng 81:103407
Askari M, Aliofkhazraei M, Afroukhteh S (2019) A comprehensive review on internal corrosion and cracking of oil and gas pipelines. J Nat Gas Sci Eng 71:1
Askari M, Aliofkhazraei M, Ghaffari S, Hajizadeh A (2018) Film former corrosion inhibitors for oil and gas pipelines-A technical review. J Nat Gas Sci Eng 58:92–114
B.M. Miksic, A.Y. Furman, M.A. Kharshan, Effectiveness of the corrosion inhibitors for the petroleum industry under various flow conditions, in: CORROSION 2009, NACE International, (2009) NACE-09573.
C.D. Mackenzie, V. Magdalenic, E. Perfect, M. Achour, D.J. Blumer, M.W. Joosten, M. Rowe, Development of a New Corrosion Management Tool-Inhibitor Micelle Presence as an Indicator of Optimum Dose, in: SPE International Conference on Oilfield Corrosion, Society of Petroleum Engineers, (2010) SPE 130285.
D. Glasgow, A. Kirkpatrick, N. Obeyesekere, M. Reid, J.J. Wylde, When To Batch and When Not To Batch: An Overview of Integrity Management and Batch Corrosion Inhibitor Testing Methods and Application Strategies, in: SPE International Symposium on Oilfield Chemistry, Society of Petroleum Engineers, (2013) SPE 164080.
Davoudi M, Heidari Y, Safadoost A, Samieirad S (2014) Chemical injection policy for internal corrosion prevention of South Pars sea-pipeline: a case study. J Nat Gas Sci Eng 21:592–599
E. Dayalan, F. De Moraes, J.R. Shadley, E.F. Rybicki, S.A. Shirazi, CO2 corrosion prediction in pipe flow under FeCO3 scale-forming conditions, in: CORROSION 98, Nace International, (1998) NACE-98051.
Finšgar M, Jackson J (2014) Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: a review. Corros Sci 86:17–41
Garcia-Arriaga V, Alvarez-Ramirez J, Amaya M, Sosa E (2010) H 2 S and O 2 influence on the corrosion of carbon steel immersed in a solution containing 3M diethanolamine. Corros Sci 52:2268–2279
Ghareba S, Omanovic S (2010) Interaction of 12-aminododecanoic acid with a carbon steel surface: towards the development of ‘green’corrosion inhibitors. Corros Sci 52:2104–2113
J.E. Wong, N. Park, Further investigation on the effect of corrosion inhibitor actives on the formation of iron carbonate on carbon steel, in: CORROSION 2009, NACE International, (2009) NACE-09569.
J.R. Davis, Corrosion: understanding the basics, ASM International, (2000) 1–17.
Javidi M, Chamanfar R, Bekhrad S (2019) Investigation on the efficiency of corrosion inhibitor in CO2 corrosion of carbon steel in the presence of iron carbonate scale. J Nat Gas Sci Eng 61:197–205
Jing J, Xiao F, Yang L, Wang S, Sun J (2018) Experimental and simulation study of atomization concentration of corrosion inhibitor in a gas pipe. J Nat Gas SciEng 49:8–18
Keewan M, Banat F, Alhseinat E, Zain J, Pal P (2018) Effect of operating parameters and corrosion inhibitors on foaming behavior of aqueous methyldiethanolamine solutions. J Petrol Sci Eng 165:358–364
Kermani M, Morshed A (2003) Carbon dioxide corrosion in oil and gas production—a compendium. Corrosion 59:659–683
Kermani M, Smith L (1997) CO2 corrosion control in oil and gas production-Design considerations. European Federation of Corrosion Publication, London (UK), Institute of Materials, p 53
M.A. Kelland, Production chemicals for the oil and gas industry, CRC press, Taylor & Francis (2014) 191–211.
Martínez D, Gonzalez R, Montemayor K, Juarez-Hernandez A, Fajardo G, Hernandez-Rodriguez M (2009) Amine type inhibitor effect on corrosion–erosion wear in oil gas pipes. Wear 267:255–258
N.G. Park, L. Morello, G. Abriam, Understanding Inhibition of Sour Systems with Water Soluble Corrosion Inhibitors, in: CORROSION 2009, NACE International, (2009) NACE-09362.
Obot I, Onyeachu IB, Umoren SA, Quraishi MA, Sorour AA, Chen T, Aljeaban N, Wang Q (2020) High temperature sweet corrosion and inhibition in the oil and gas industry: Progress, challenges and future perspectives. J Petroleum Sci Eng 185:106469
Popoola LT, Grema AS, Latinwo GK, Gutti B, Balogun AS (2013) Corrosion problems during oil and gas production and its mitigation. Int J Ind Chem 4:1–15
R. Hill, F. Ramirez, B. Monty, G. Palanivel, Material selection assessment for topside process equipment for production fluids with high CO2 content, in: CORROSION 2011, NACE International, (2011) NACE-11118.
R. Nyborg, Pipeline corrosion prevention by ph stabilization or corrosion inhibitors, in: Rio Pipeline Conference and Exposition, Brazilian Petroleum, Gas and Biofuels Institute-IBP Rio de Janeiro, Brazil, (2009) IBR1527_09.
R.D. Tems, A.M. Al-Zahrani, Cost of corrosion in gas sweetening and fractionation plants, in: CORROSION 2006, NACE International, (2006) NACE-06444.
Rahuma MN (2014) Corrosion in oil and gas industry: a perspective on corrosion inhibitors. J Mater Sci Eng 3:1
S. Papavinasam, Corrosion control in the oil and gas industry, Elsevier, (2013) 249–297.
S. Ramachandran, K.A. Bartrip, Molecular Modeling of Binary Corrosion Inhibitors, in: CORROSION 2003, NACE International, (2003) NACE-03624.
S. Stewart, C. Menendez, V. Jovancicevic, J. Moloney, New Corrosion Inhibitor Evaluation Approach for Highly Sour Service Conditions, in: CORROSION 2009, NACE International, (2009) NACE-09360.
T. das Chagas Almeida, M.C.E. Bandeira, R.M. Moreira, O.R. Mattos, New insights on the role of CO2 in the mechanism of carbon steel corrosion, Corrosion Science, 120 (2017) 239–250.
T.J. Mesquita, H. Marchebois, Key Materials in Oil and Gas Production and the Choice of Inhibitors, Corrosion Inhibitors in the Oil and Gas Industry, (2020) 111–133.
Trabanelli G (1991) Whitney award lecture: inhibitors-an old remedy for a new challenge. Corrosion 47(1991):410–419
Videm K, Koren A (1993) Corrosion, passivity, and pitting of carbon steel in aqueous solutions of HCO3-, CO2, and Cl. Corrosion 49:746–754
Acknowledgements
The authors gratefully thank the support of Sonatrach Company to enable us to carry out this work.
Funding
I declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gharbi, K., Chouicha, S. & Kelland, M.A. Field test investigation of the performance of corrosion inhibitors: a case study. J Petrol Explor Prod Technol 11, 3879–3888 (2021). https://doi.org/10.1007/s13202-021-01287-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-021-01287-y