Abstract
The oil reservoir pressure declines due to oil production, and this decline will lead to reduction in the oil productivity. The reservoir pressure maintenance is a practice in the oil industry in which seawater is injected into the aquifer zone below the oil zone to support the reservoir pressure. Calcium sulfate scale is one of the most serious oilfield problems that could be formed in sandstone and carbonate reservoirs. Calcium sulfate may precipitate during the injection of seawater with high sulfate content into formation brine with high calcium content. Mixing seawater and formation water may cause precipitation of calcium sulfate, barium sulfate, and/or strontium sulfate. Seawater treatment does not remove the entire sulfate ions from the injected water. Low sulfate concentrations may cause damage. Enhanced oil recovery processes such as smart water injection, which originally is diluted seawater, may cause calcium sulfate precipitation as the reduction of water salinity will increase the sulfate precipitation and decrease its solubility. This study was conducted to investigate the damage caused by the deposition of calcium sulfate precipitation. A solution is proposed to prevent the damage due to calcium sulfate by using chelating agents. Several coreflooding experiments were conducted using Berea sandstone and Indiana limestone cores at reservoir conditions of pressure and temperature using seawater (high and low salinity) and formation water. Chelating agents used in this study are: EDTA (ethylenediaminetetraacetic acid), HEDTA (hydroxyethylenediaminetriacetic acid), and HEIDA (hydroxyethyliminodiacetic acid). HEDTA and HEIDA chelating gents are environmentally friendly and can be used in marine environment. High-salinity water injection caused severe formation damage, and the injectivity will decline faster compared to the low-salinity water injection. HEDTA and EDTA chelating agents at low concentrations performed better than HEIDA chelating agents in both Berea sandstone and Indiana limestone cores. HEDTA and EDTA chelating agents were able to prevent the damage due to calcium sulfate precipitation and enhanced the core permeability.
Similar content being viewed by others
Introduction
The injection of modified seawater or low-salinity water increased the oil recovery from carbonate and sandstone reservoirs (McGuire et al. 2005). Low-salinity water gave better recovery than seawater because of the low salt concentration of the low-salinity water (Morrow et al. 1998; Tang and Morrow 1999a; 1999b; 1997). Lowering the salinity or total dissolved solids (TDS) of the injected water resulted in reducing the oil–rock capillary pressure, decreased the oil–water interfacial tension, and changed the wettability that caused relative permeability change (Tang and Morrow 1997).
Low-salinity water flooding might cause formation damage in both sandstone and carbonate reservoirs. The formation damage in sandstone reservoir will be due to clay fines migration that might block the pore throats and reduce the permeability. Usually, carbonate formations contain high-salinity brines with high calcium concentration (up to 20,000 ppm) and this will cause calcium sulfate precipitation under the reservoir conditions of high pressure and temperature because the low-salinity water contains sulfate ions (Mahmoud 2014).
Surfactants and polymers are used extensively in enhanced oil recovery to recover more oil from sandstone and carbonate reservoirs. Son et al. (2013) used nanoparticles to stabilize oil/water emulsions for enhanced oil recovery. They found out that the stabilized emulsion by nanoparticles yielded 11 % oil recovery after seawater flooding. The recovery increase was attributed to the piston-like displacement achieved by the high-viscosity emulsion. Fu et al. (2013) used novel cationic starch polymer for enhanced oil recovery. They found out that the cationic starch enhanced the oil recovery and reduced the water cut and yielded better results than other commercial polymers used in the enhanced oil recovery processes.
A common issue in oil and gas production is scale deposition that ends up with permeability reduction in the near-wellbore area. Millions of dollars are spent every year to remove formation damage in the near-wellbore area of the producing gas and oil wells. Seawater is injected into oil-bearing reservoirs to maintain the reservoir pressure and improve secondary recovery. The incompatibility between the injected seawater and the formation brine causes precipitation of inorganic scale in surface equipment, flow lines, well tubing, gravel pack, and inside the reservoir. The precipitation in the reservoir is the most severe problem and most expensive one to solve. Seawater contains more than 2800 ppm sulfate, and the reservoir water contains usually high levels of barium, strontium, and calcium; therefore, there is a high tendency to form sulfate-based scales. Typical sulfate scales that may arise from water mixing include barite (BaSO4), celestite (SrSO4), and anhydrite (CaSO4). Mineral scale precipitation will occur due to the mixing between the injected seawater and the formation water (Atkinson et al. 1991). The precipitation of sulfate scale can greatly reduce the formation permeability and in turn the injectivity of the injected water.
The solubility product constants (Ksp) for different carbonate and sulfate scales decrease with temperature for a temperature range of 60–300 °F (Nassivera and Essel 1979; Essel and Carlberg 1982; Shen and Corsby 1983).
Oddo et al. (1991); Oddo and Tomson (1994) showed that the formation of calcium sulfate scale depends on temperature and does not depend on pH and can precipitate at low as well as high pH values. In the case where water injection (seawater, river, aquifer, or produced water) is used for pressure maintenance and sweep, the mixing of incompatible aqueous solutions can lead to the formation of sulfate scales when the injection water contains sulfate ions (Mackay and Jordan 2005). Nassivera and Essel (1979) showed that the solubility of gypsum and anhydrite is a strong function of temperature and it decreases with increasing the temperature.
Chelating agents contain different functional groups (carboxyl, hydroxyl, ether, primary amine, tertiary amine, thiol, nitro, nitroso, and sulfine, etc.) which have the ability of grabbing the metal ion and form a stable complex. Dissociated carboxyl group turns out to be the best sequestering group. Tertiary amine is the most promising group among the neutral groups (Bakken and Schöffel 1996). The structures of chelating agents are typically represented by HnY where the n hydrogen’s are those of the carboxylic acid groups. The conjugate bases of the chelating agents have the ability to chelate different ions such as iron and calcite, which present in reaction solutions. The affinity of conjugate base (ligand), An−, for different ions, Mm+, is dependent on stability of the formed conjugate base and ion complex molecule, and the chelation ability.
Formation damage due to low salinity enhanced oil recovery in carbonate reservoirs
Zahid et al. (2012) show the pressure drop profile versus injection rate during low- and high-salinity seawater injection through oil-saturated limestone cores. Diluting seawater ten times increased the pressure drop 10 times at 0.1 cc/min injection rate and five times at 0.5 and 1 cc/min injection rates. Diluting seawater should decrease its viscosity because its density goes down with dilution. The increase in the pressure drop when using low-salinity diluted seawater instead of seawater can be attributed to the calcium sulfate precipitation and the capillary pressure effects. Carlberg and Matthews (1973) show the relation between water salinity and calcium sulfate solubility in the water. Diluting seawater ten times will decrease the calcium sulfate solubility in the water, the precipitation rate of calcium sulfate will increase, and in turn, the pressure drop will increase. The pressure drop increase ratio was low at higher injection rate due to the low contact time between the injected water and brine; therefore, the precipitation rate of calcium sulfate will be lower at injection rates 0.5 cc/min compared to that at 0.1 cc/min. From the previous work, we can conclude that low-salinity water injection was good in oil recovery, but it precipitated calcium sulfate and this will reduce the water injectivity. The solution of this problem is to add chelating agents to the injected water at low concentrations on a slug mode to prevent the sulfate scale precipitation. Chelating agents such as EDTA, HEDTA, HEIDA, MGDA, GLDA, NTA, and DTPA can be used for this purpose. Chelating agents should be used at high pH to avoid corrosion and compatibility problems with the seawater and formation brine.
The objectives of this paper are to: (1) investigate experimentally the effect of the injected seawater (high salinity and low salinity) on the reservoir permeability, (2) use EDTA, HEDTA, and HEIDA chelating agents to prevent the precipitation of calcium sulfate scale due to seawater injection, and (3) investigate the effect of using chelating agents on the reservoir permeability.
Experimental studies
Materials
The chelating agents used in this study are EDTA, HEDTA, and HEIDA. The original concentration of these chelating agents was 40 wt% at pH = 11. The concentrations of the chelating agent used in the study were 1, 5, and 10 wt%, and the dilution was done using high-salinity seawater and low-salinity water with the composition listed in Table 1. Berea sandstone and Indiana limestone cores of dimensions 1.5 in × 6 in. were used in the flooding experiments. The mineralogy of the Berea sandstone cores is listed in Table 2. All cores were saturated using formation brine (connate water) of composition shown in Table 1. The cores were saturated by high-pressure saturator (pressure = 1000 psi) after vacuum for 24 h.
Experimental procedure
The coreflooding experiments were performed using the coreflooding setup shown in Fig. 1. A back pressure of 1000 psi was applied in all experiments. One back pressure regulator at the outlet of the core holder. A hydraulic pump was used to apply the required confining pressure on the core. High-accuracy pressure transducers were used to measure the pressure drop (accuracy = 0.02 psi) with the range of 0–1000 psi. The core flood tests were carried out at 100 °C. Before running the core flood experiment, the core was first saturated with brine and the pore volume was calculated. In each coreflooding experiment, the core was first loaded into the core holder at an overburden pressure 500 psi more than the inlet pressure was applied at 100 °C. Then, it was saturated with injection water until the brine permeability became constant. The brine used in the experiments was formation brine, as shown in Table 1 (connate water composition).
Results and discussion
Chelating agent diluted with high-salinity water
Figure 2 shows the damage caused after injecting seawater and low-salinity water with the salinity listed in Table 1. Four different coreflooding experiments were carried out at 100 °C and 0.25 cm3/min and at 1000 psi back pressure. The damage caused by the seawater was higher compared to that caused by the low-salinity water. The cores were saturated by connate water that contains more than 19,000 ppm calcium. The sulfate from the injected water reacted with the calcium from the connate water and precipitated calcium sulfate inside the core, and because of that, the permeability decreased. The permeability reduction was higher in the case of seawater because it contains more sulfate compared to the low-salinity water. For the Indiana limestone cores, the seawater caused permeability reduction of 28 % and the low-salinity water caused 13 % reduction in the core permeability. In the case of Berea sandstone cores, similar trends of permeability reduction were obtained for the seawater and low-salinity waters. The seawater injection caused 19 % reduction in the permeability and the low-salinity water caused 7 % reduction in the case of Berea sandstone cores. The reduction in the permeability of Berea sandstone cores was lower compared to Indiana limestone cores because of the higher permeability of the Berea sandstone cores. The average permeability of Berea sandstone cores was 80 md, and the average permeability of the Indiana limestone cores was 5 md. The low-permeability cores have small pore-throat size, and this will promote the damage due to the restriction of the movement of the formed calcium sulfate crystals. The calcium sulfate precipitation highly damaged the core during seawater injection because of the high sulfate content. The dilution of seawater reduced the sulfate concentration from 4290 to 1073 ppm. Comparing the ratio of calcium sulfate in the low-salinity and high-salinity water from 4290 to 1073, the factor of dilution for sulfate was four, which is the main source of damage. Naturally, we should expect the same in calcium sulfate precipitation reduction, which is not the case. The sulfate concentration of 4290 ppm caused 28 % loss in the core permeability, reducing the sulfate concentration to 25 % of its original concentration should reduce the damage from 28 to 7 %. The permeability reduction in the low-salinity water (low sulfate content) was 13 % compared to 28 % in the high-salinity water. This can be attributed to that the calcium sulfate solubility is also affected by sodium chloride concentration and it goes down when the sodium chloride concentration is decreased.
The experimental results shown in Fig. 3 confirmed the effectiveness of different chelating agents in preventing the precipitation of calcium sulfate scale during seawater injection. Figure 3 shows the effect of EDTA concentration on the permeability of Berea sandstone cores. Low concentration of EDTA (1 wt%) was only able to prevent the sulfate precipitation and did not chelate any cations from the core; the permeability of the core remained the same before and after seawater injection. The core initial permeability was 80 md, and the final permeability after seawater injection was 79 md. Increasing the concentration of EDTA to 5 wt% prevented sulfate precipitation by chelating calcium in solution and enhanced the core permeability from 73 to 82 md (Kfinal/Kinitial = 1.125). The 5 wt% concentration of EDTA chelated other cations such as Mg2+ and Fe3+ form the core. Increasing the concentration to 10 wt% enhanced the permeability better than 5 wt% as more cations were chelated from the core (Ca2+ from calcite and dolomite, Mg2+ from dolomite, and Fe3+ from chlorite); the permeability improvement ratio (final permeability/initial permeability) was 1.3.
The same set of experiments was performed using different concentrations of HEDTA chelating agents at the same conditions used for EDTA, as shown in Fig. 3. The performance of HEDTA almost was almost the same as that of EDTA in preventing sulfate scale precipitation and in enhancing the sandstone core permeability. EDTA and HEDTA have the ability to chelate calcium, magnesium, aluminum, and iron from Berea sandstone cores. The iron can be chelated from chlorite mineral, calcium from calcite and dolomite, magnesium can be chelated from dolomite, and aluminum can be chelated from clay minerals such as illite and kaolinite. Both EDTA and HEDTA are compatible with Berea sandstone cores, and no fines were observed in the collected effluent samples (Mahmoud et al. 2015; 2011).
Figure 3 shows the effect of using HEIDA chelating agent on sulfate scale precipitation and on the enhancement of core permeability. As shown in this figure, HEIDA at 1 wt% concentration was not able to prevent the damage due to calcium sulfate precipitation (permeability ratio = 0.8). HEIDA chelating agent should be used at higher concentration to prevent the precipitation of sulfate scale such as 5 and 10 wt%.
Figure 4 shows the effectiveness of using EDTA, HEDTA, and HEIDA chelating agents in preventing sulfate scale precipitation and enhancement of core permeability in Indiana limestone cores. EDTA was the best chelating agent in preventing the damage and enhancing the core permeability, and HEIDA was the weakest chelate among the three chelates used in this study. Generally, EDTA, HEDTA, and HEIDA chelating agents at high concentration (10 wt%) performed better in carbonate cores because of their high chelation ability of calcium. EDTA at 1 wt% concentration performed the same in sandstone and carbonate, and it was able only to chelate the calcium from the formation brine and the seawater. Increasing the concentration to 5 and then 10 wt% made the EDTA chelating agent more powerful, and it was able to dissolve calcite in carbonate cores more than sandstone cores (low carbonate concentration, 2 wt%). The performance of EDTA was better in Indiana Limestone cores because of its good ability to chelate calcium.
Chelating agents diluted with low-salinity water
Figures 5 and 6 show the effect of using EDTA, HEDTA, and HEIDA chelating agents diluted in low-salinity water in preventing calcium sulfate scale damage in Indiana limestone and Berea sandstone cores. The final concentration of chelating agents was obtained from an initial concentration of 40 wt%, and it was diluted to the required concentration using low-salinity water having a composition listed in Table 1. All chelating agents performed better when diluted with low-salinity water compared to that diluted with seawater. Seawater has high sodium chloride content, and this affected the stability of chelating agents. Chelating agents with high sodium chloride concentration has low dissolving power for different minerals compared to chelating agents with low sodium chloride concentration (Mahmoud et al. 2011). Reducing sodium chloride concentration allowed the chelating agents to chelate more calcium from the solution and from the rock; therefore, the permeability enhancement was higher in the case of chelating agents diluted with low-salinity water compared to that diluted with high-salinity seawater. For example, EDTA chelating agent at 5 wt% concentration enhanced the permeability of Indiana limestone cores by 65 % when diluted in low-salinity water compared to 25 % for EDTA when diluted in seawater.
Effect of pH on the compatibility between chelating agents and seawater
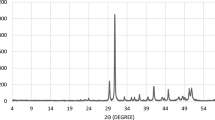
Chelating agents’ compatibility with seawater was found to be a strong function of pH value. Table 3 and Fig. 7 show the effect of EDTA pH on the compatibility of EDTA and seawater. The five solutions were prepared from an initial solution of H2Na2EDTA of pH value 4.36. Potassium hydroxide was used to increase the pH. As shown in Fig. 7, there was a white precipitate at the first three solutions (pH = 4.36, 5.26, and 6.34), and XRD showed that it is an organic precipitate with slight fraction of calcium sulfate. Increasing the pH value to 7.2 made the EDTA compatible with seawater, and no precipitation was observed. EDTA cannot be used with seawater at low pH values; it should be used at pH values >7 if there is an essential need to mix it with seawater. EDTA with deionized and fresh water did not precipitate at pH values >4.36.
Figure 8 shows two different coreflooding experiments at two different EDTA pH values. The first experiment was performed using 5 wt% EDTA chelating agent at pH value of 6, which is in the insoluble range (less than 7). The permeability of the Indiana limestone core decreased by 5 % because of the EDTA precipitation inside the core, and this confirmed the solubility results shown in Table 3 and Fig. 7. When we used EDTA at pH value of 11 the Indiana limestone core permeability increased by 25 %. The coreflooding experiments results were consistent with the solubility experiments.
Conclusions
Calcium sulfate scale precipitation affected the reservoir permeability and porosity. In this study, we performed several coreflooding experiments to investigate the effect of HEDTA, HEIDA, and EDTA chelating agents to prevent and remove the damage due to calcium sulfate scale from carbonate and sandstone cores. The following are the conclusions that were drawn from this study:
-
1.
EDTA and HEDTA chelating agents at different concentrations were able to chelate all calcium from the solution and prevent the precipitation of calcium sulfate scale in calcite and sandstone cores.
-
2.
Higher concentrations (5 and 10 wt%) of HEDTA and EDTA enhanced the permeability of carbonate and sandstone cores more than low concentration (1 wt%).
-
3.
HEIDA chelating agent at 1 wt% concentration was not effective in preventing the calcium sulfate precipitation. HEIDA should be used at concentrations higher than 5 wt% to prevent sulfate scale precipitation in both calcite and sandstone cores.
-
4.
Low-salinity water injection precipitated calcium sulfate because of the low solubility of calcium sulfate in low-salinity water, but the damage was lower than that caused by high-salinity seawater.
-
5.
Chelating agents diluted in low-salinity water performed better than that diluted in high-salinity seawater in removing the damage from both sandstone and carbonate cores.
Abbreviations
- BaSO4 :
-
Barium sulfate
- CaSO4 :
-
Calcium Sulfate
- DTPA:
-
Diethylenetriamine pentaacetic acid
- EDTA:
-
Ethylenediaminetetraacetic acid
- GLDA:
-
Glutamic diacetic acid
- HEDTA:
-
Hydroxyethylenediaminetriacetic acid
- HEIDA:
-
Hydroxyethyliminodiacetic acid
- Kfinal :
-
Final permeability
- Kinitial :
-
Initial permeability
- Ksp :
-
Solubility product constant
- MGDA:
-
Methylglycinediacetic acid
- NTA:
-
Nitrilotriacetic acid
- PV:
-
Pore volume of the core
- SrSO4 :
-
Strontium sulfate
References
Atkinson G, et al. (1991) The thermodynamics of scale prediction. Paper SPE 21021 presented at the international symposium on oilfield chemistry held in Anaheim, California, 20–22 February 1991
Bakken V, Schöffel K (1996) Semi-quantitative study of chelating agents suitable for removal of scales. Oil Gas Sci Technol J 51(1):151–160
Essel JA, Carlberg LB (1982) Strontium sulfate scale control by inhibitor squeeze treatment in the fateh field. J Pet Technol 34(6):1302–1306
Fu J, Qiao R, Zhu L, Zhu W, Hao S (2013) Application of a novel cationic starch in enhanced oil recovery and its adsorption properties. Korean J Chem Eng 30(1):82–86
Mackay JE, Jordan MM (2005) Impact of brine flow and mixing in the reservoir on scale control risk assessment and subsurface treatment options: case histories. J Energy Res Technol 127(3):201–213
Mahmoud MA (2014) Evaluating the damage due to calcium sulfate scale precipitation during low and high salinity water injection. J Can Pet Technol 53(3):141–150
Mahmoud MA, Nasr-El-Din HA, DeWolf CA (2011) Novel environmentally friendly fluids to remove carbonate minerals from deep sandstone formations. Paper SPE 143301 presented at the SPE European Formation Damage Conference, Noordwijk, The Netherlands, 7–10 June 2011
Mahmoud MA, Nasr-El-Din HA, DeWolf CA (2015) High-temperature laboratory testing of illitic sandstone outcrop cores with HCl-alternative fluids. SPE Prod Oper J 30(1):43–51
Carlberg BL, Matthews, RR (1973) Solubility of calcium sulfate in brine. Paper SPE 4353 presented at the oilfield chemistry symposium, Denver, Colorado, 24–25 May 1973
McGuire PL, Chatham JR, Paskvan FK, Sommer DM, Carini FH (2005) Low salinity oil recovery: an exciting new EOR opportunity for Alaska’s North slope. Paper SPE 93903, presented at the SPE Western Regional Meeting, Irvine, California, 30 March–1April 2005
Morrow NR, Tang G, Valat M, Xie X (1998) Prospects of improved oil recovery related to wettability and brine composition. J Pet Sci Eng 20(3–4):267–276
Nassivera M, Essel A (1979) Fateh field sea water injection—water treatment, corrosion, and scale control. Paper SPE 7765 presented at the Middle East Oil Technical Conference, Manama, Bahrain, 25–29 March 1979
Oddo E J, Smith P J, Tomason B M (1991) Analysis of and solutions to the CaCO3 and CaSO4 scaling problems encountered in wells offshore Indonesia. Paper SPE 22782 presented at the ATCE, Dallas, TX, 6–9 October (1991)
Oddo JE, Tomson MB (1994) why scale forms in the oil field and methods to predict it. SPE Prod Facil J 9(1):47–57
Shen J, Corsby CC (1983) Insight into strontium and calcium sulfate scaling mechanisms in a wet producer. J Pet Technol 35(7):1249–1255
Son H, Kim H, Lee G, Kim J, Sung W (2013) Enhanced oil recovery using nanoparticle-stabilized oil/water emulsions. Korean J Chem Eng 31(2):338–342
Tang GQ, Morrow NR (1997) Salinity, temperature, oil composition, and oil recovery by waterflooding. SPE Reserv Eng J 12(4):269–276
Tang G, Morrow NR (1999a) Influence of brine composition and fines migration on crude oil/brine/rock interactions and oil recovery. J Pet Sci Eng 24(2–4):99–111
Tang G, Morrow NR (1999b) Oil recovery by waterflooding and imbibition-invading brine cation and salinity. Paper SCA9911 presented at the International SCA Symposium held in Golden, Colorado, 1–4 August 1999b
Zahid A, Shapiro A, Skauge A (2012) Experimental studies of low salinity water flooding carbonate: A new promising approach. Paper SPE 155625, presented at the EOR conference at Oil and Gas west Asia, Muscat, Oman, 16–18 April 2012
Acknowledgments
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, through the Science and Technology Unit at King Fahd University of Petroleum and Minerals (KFUPM), The Kingdom of Saudi Arabia, award number (13-Oil-151-04).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mahmoud, M., Elkatatny, S. & Abdelgawad, K.Z. Using high- and low-salinity seawater injection to maintain the oil reservoir pressure without damage. J Petrol Explor Prod Technol 7, 589–596 (2017). https://doi.org/10.1007/s13202-016-0279-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-016-0279-x