Abstract
The microelectronics industry wastewater is attracting the scientific community and industry attention due to the high amount of organic and inorganic pollutants produced. Pollutants in microelectronic wastewater are considered qualitative contaminants, found in low concentrations but present with high risks to the environment and public health. The current paper highlights the most common pollutants in microelectronics wastewater and discusses the recent treatment technologies used to remove these contaminates. The review process was conducted based on the identification and screening of microelectronic wastewater and the principle of contaminant detection reported in previous research literature. It appears that tetramethylammonium hydroxide and ammonium are the major organic compounds in microelectronic wastewater, while heavy metals are among the inorganic pollutants with high environmental toxicity. The membrane filtration is the most efficient method for removing the pollutants (more than 90%) and producing ultrapure water. However, wastewater should be subjected to primary treatment using chemical and biological methods before undergoing the ultrafiltration process. The zero-discharge concept is more applicable in the microelectronics industries due to the stringent regulations associated with these industries. Microelectronic sludge represents the main challenge in wastewater recycling, while advanced treatment methods have been suggested for sludge treatment. More effort is required to recycle microelectronics owing to the high prices related to the disposal of these solids’ wastes.
Similar content being viewed by others
Introduction
With the high demand for electronics integrated circuits for various industries, microelectronics is among the industries with the highest growth and the production speed is increasing exponentially. Semiconductors’ applications include robots, artificial intelligence, displays, smart factories, and smart cars. The toxicity of chemical substances used in the semiconductor manufacturing process is associated with several risks to mankind and the environment (Mori et al. 2015). Semiconductor wastewater is one of the complex industrial effluents and represents 28% of the total wastewater discharged into the environment without treatment (Mao et al. 2022). These wastes have received significant attention in terms of zero-liquid discharge and resource recovery among researchers. The main challenge lies in the composition of variants in the generated effluents (Cielik and Konieczka 2017).
Electronic waste (E-waste) is a class of waste categorised for the microelectronics industries, but these wastes can be generated from manufacturing and households. As reported on Digital 2020 (Kemp 2020), around 5.15 billion mobile phone consumers are worldwide, confirming the precipitous development of electronic products worldwide. Electronic waste in 2019 has been reported to be 53.6 million metric tons and is expected to reach 74.7 million metric tons by 2030 (Forti et al. 2020). Europe produces 16.2 kg per capita of e-waste, while 13.3 kg per capita is generated in America and 5.6 kg per capita in Asia. Japan is the only country which has implemented extended producer responsibility (EPR) (Rajesh et al. 2022). Figure 1 shows the trends of scientific publications associated with the microelectronics industries (using specific keywords (“microelectronics” AND “wastewater” OR “sludge” AND “Treatment technologies”), which show that these industries have increased during the years and are expected to increase more in the future. According to Fig. 2, among the countries with high microelectronics activity are the USA (32.7%), India (9%), and Canada (8%). Taiwan will account for 26% of semiconductor sales in 2021 and is the second-largest market in the world, with 64% of the foundry market. Currently, Taiwan’s 24 manufacturing plants are the major microelectronics production group, followed by China, South Korea, and the USA (Trend Force 2022).
The purpose of the present paper is to review the opportunities of microelectronic wastewater treatment to overcome the issues associated with environmental, technology, regulatory, and economics. Based on the review process of the scientific publication on microelectronic wastewater treatment, very few studies have addressed the current technologies and highlighted the challenges associated with the treatment process of such wastewater. The present work reviewed the most advanced technologies used in the treatment process, such as plasma, membrane, and ultrahigh purification membranes. The main pollutants in microelectronic wastewater were reviewed, while the applicability of recovering and recycling some materials from microelectronic wastewater was discussed. The present work provided more information on the quantitative and qualitative pollutants in microelectronic wastewater, focusing on the efficiency of different treatment technologies currently used to recover metals and eliminate organic compounds. The readers will be provided with detailed information on the applicability of the biological, chemical, and physical treatment of microelectronic wastewater. Recycling this wastewater into pure water to be reused in the industry process is the highlight of this review paper.
Review methodology
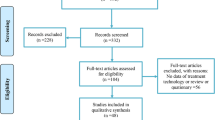
The review method employed in this study was initiated by searching relevant documents from Scopus databases based on the specific keywords, including “microelectronics” AND “wastewater” OR “sludge” AND “Treatment technologies”. The screening process was conducted according to the review process described by Haddaway et al. (2022) (Fig. 3). The screening process included the review of the title and abstract of each research paper downloaded. Each research paper with a clear title and abstract related to microelectronics wastewater was selected for further reading on the methodology and results. The database obtained from Scopus was analysed by bibliometric analysis using the VOSviewer software tool to find the most common countries with microelectronics industry activities, a list of the heavy metals used in the microelectronics process, as well as a list of agriculture adsorbents used for the expulsion of heavy metals. During the review process, the list of pollutants in the microelectronics wastewater was listed.
Microelectronics wastewater characteristics
The waste contribution of the microelectronics industry, leading to adverse environmental pollution through the production of huge quantities of wastewater containing fluoride, phosphate, and heavy metals, is apprehensive. Table 1 summarises the physicochemical characteristics of microelectronic wastewater reported from different studies. Figure 4 shows the most common type of pollutants from microelectronic waste. Figure 5 shows that the most intensively used heavy metals in the microelectronics industries are silver (27.38%), silica (26.19%), and copper (14.29%). Silica (Si) residues are among the metals in the effluents of the microelectronics industry that can be recovered and recycled. Gallium (Ga), molybdenum (Mo), lead (P), boron (B), and arsenic (As) are utilised to fabricate semiconductors (Schischke et al. 2001). In the microelectronics sludge, the Si was found with a concentration between 500 and 2000 mg/L (Belongia et al. 1999). The recovery of Si particles enables recycling of semiconductor manufacturing processes or conversion of them to useful resources (Teow et al. 2022).
Wu et al. (2012) revealed that Si, vanadium (V), selenium (Se), strontium (Sr), and uranium (U) metals were among the metal pollution in the river contaminated with effluent plants from a microelectronic industry. Tungsten concentrations in streams contaminated with treated wastewater from Taiwan’s semiconductor manufacturer were 400 μg/L in water samples and 300 μg/g in sediments, which exceeded the standard concentration of < 0.1 μg/L (Hsu et al. 2011). A study carried out on the accumulation in river waters and bed sediments contaminated with effluents from semiconductor industries revealed that Pb2+, Cr2+, Cd2+, Zn2+, and Ni2+ were the most common elements found (Hsu et al. 2016). Extreme amounts of ammonium (NH4) and phosphate (PO4) were reported in the semiconductor wastewater (Ryu et al. 2012a, b). The fluoride compound in semiconductor wastewater was discovered to range from 250 to 1500 mg L−1 and was generated from the etching process involved in the integrated circuit chip fabrication (Liu and Liu 2016; Lacson et al. 2021).
The wastewater generated from the microelectronics process has less organic matter content, such as phosphoric acid, organic solvents, and NH4OH (Bang et al. 2016). These pollutants are generated using raw substances during manufacturing, including subprocesses (such as cleaning), wet etching, photolithography, backgrinding, and dicing (Innocenzi et al. 2022). Tetramethylammonium hydroxide (TMAH, C4H13NO) is among the chemical substances with highly problematic compounds generated by photolithography (Chung et al. 2020a, b). Diluted backgrinding wastewater (DBGW) generated from the backgrinding process is characterised by alkaline pH, NH3–N, and high colour saturation (Yang et al. 2012). Chemical mechanical polishing wastewater (CMPW) is the waste generated by the chemical mechanical polishing process (CMP). The surface of the semiconductor is made smooth and uniform by applying a mixture of abrasives like SiO2, Al2O3, or CeO2 alongside the chemical oxidation process, which exerts a downward force on the slurry (Fatehah et al. 2013). Each process produces a different type of wastewater with different characteristics. It is challenging to provide an accurate composition of the microelectronic effluent. The first step used in the treatment of microelectronics is focusing on separating the wastewater based on the source.
Based on the previous studies, microelectronic wastewater greatly differs from other industrial wastewater. These wastewaters contain between 0.34 and 3.35 mM of perfluorooctanoic acid (PFOA) in wastewater generated from the emulsifying process in fluoropolymer manufacturing, and between 5 and 60 g/L of TMAH, 5 and 66 g/L of glycerol, 50–2000 mg/L of pyrazole, 100–200 mg/L of acetone, 700–800 mg/L of calcium fluoride (CaF2), 50–1000 mg/L of KOH, NH3–N 2.8–3.9 mg/L, turbidity (NTU) 1044–6390, with pH ranging from 6.3 to 9.8, 0.1–5.3 g/L of total solid, 0–0.2 mg/L of suspended solid, 175–5000 mg/L of COD, BOD/COD between 0.11 and 0.15, 45.6–58.2 mg/L of sulphates, 65.38–72.58 mg/L of total Kjeldhal nitrogen (Omar et al. 2013; Post et al. 2012; Wang et al. 2022; Teow et al. 2022). The main pollutants of organic solvent particles range from nano- to micro-sized (Omar et al. 2013). The contaminants are available in very low concentrations. However, it is a complex material that is difficult to degrade and eliminate using traditional treatment methods.
Microelectronics effluent treatment and filtering technology
The microelectronic process represents one of the largest water consumptions. Manufacturing with a huge, consolidated circuit needs around 8328 L of water in total (for how long and how many industries, this is a fuzzy figure), of which 5678 L are ultrapure water (UPW). In 2006, 26,000 tons of wastewater were generated daily in Taiwan from the integrated circuit (IC) industries, and the recovered sludge per year was estimated to be 24,000 tons (Lee and Liu 2009). The semiconductor industry spends more than US dollar one billion on water and wastewater treatment systems annually. A typical factory semiconductor production consumes about 40% of UPW, mostly used for washing and cleansing (Drouiche et al. 2007). UPW is required in semiconductor manufacturing to clean the surface of waters and be used in photolithography, stripping, electroplating, Si thinning, and etching (Lee et al. 2016). These UPWs should meet water quality criteria such as resistivity of more than 18.2 MΩ cm at 25 °C and total organic carbon (TOC) < 0.5 μg/L (Melnik and Krysenko 2019).
Figure 6 shows the UPW production system, which is most common in microelectronics manufacturing. The water used in the microelectronics process as well as the wastewater generated are subjected to several treatment stages, which include organic removal by the biotreatment process, continuous microfiltration for turbidity removal, selective ion-exchange for removing the ions, prefiltration: UF/MF/Media filters, continuous deionisation (cedi, ultrapure, cylindrical series), degasification using membrane technologies, ultraviolet (UV) oxidation and advanced oxidation process (AOP), ultrafiltration reverse osmosis (RO), and electrolysis have been used to improve the quality of effluents to be reused in the microelectronics process. RO is one of the UPW-producing treatment technologies because it is more efficient in reversing pollution and removing TDS and low molecular weight organic compounds (Chen et al. 2020; Wang et al. 2022). These processes are performed to produce high-quality water and achieve the concept of zero-liquid discharge. Among several contaminates in the microelectronics are phosphate, which is removed by chemical coagulation, and ammonia, which is removed by membrane bioreactors as a function of microorganisms. Inorganic contaminants are removed by adsorption as a function of ion-exchange and filtration. The reverse osmosis/deionisation (RODI) process in microelectronics wastewater is common for producing high-purity water. During the generation of ultrapure water in the microelectronics industries, one of the main issues lies in the concentration of silica in the applied waters, which varies from 1 to 300 ppm (mg/L), while in the fine microelectronic structures industries, these concentrations should be less than 1 ppb (μg/L). The trends focused on understanding silica chemistry and its role in controlling the RO process and maximising silica reduction (Ning 2003).
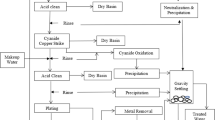
Figure 7 shows a diagram of the treatment technologies used for microelectronic wastewater. Teow et al. (2022) investigated the efficiency of ultrafiltration (UF) polymeric and ceramic membranes for the removal of Si from semiconductor-industry wastewaters, which included collection tank wastewater (CTW), CMPW, and BGW. The study showed that ceramic membranes reached the highest permeation flow (131.23–308.98 L/m2h) while having a lower relative reduction in flows indicates that they are less vulnerable to pollution (8.22–55.79%) due to their permeability, water permeability, and high porosity. The recovered Si has a high level of purity with 39.8 wt%. The removal efficiency reached more than 90% for colour, turbidity, and SS, while less than 50% for NH3–N.
In 2022, An and colleagues assessed three RO membranes (ESPA2-LD, RE4040-BE, and TMG10D) for semiconductor wastewater treatment. The wastewater was first subjected to primary treatment through ozone oxidation using a ceramic membrane before undergoing RO filtration. The study findings indicated that TMG10D had the highest permeability, followed by ESPA2-LD and RE4040-BE.
Chung et al. (2014) conducted a study on treating wastewater generated from semiconductor manufacturing, utilising a four-stage Bardenpho process in combination with a membrane system. The treatment was conducted for 14 months with a hydraulic retention time (HRT) ranging from 9.5 to 30 h. The study findings showed a 97% removal of COD and 93% removal of total nitrogen.
In another study by Dabwan et al. (2018), paper sludge ash (PSA) was utilised as an inorganic coagulant to remove colloidal silica from semiconductor wastewater. The effectiveness of the coagulant was evaluated based on dosage, pH, and temperature. The study found that the highest removal efficiency was achieved at pH 8, using 0.25 g of PSA per gram of silica at a colloidal silica concentration of 1.8% and a temperature range of 10–40 °C. Increasing the coagulant dosage at temperatures below 20 °C contributed to clearer effluent.
The process of eliminating or extracting heavy metals from wastewater produced by microelectronic devices
Onundi et al. (2010) determined the removal of Cu2+, Pb2+, and Ni2+ from synthetic semiconductor wastewater by applying palm kernel shell as the granular activated carbon as a function of time and pH. The study revealed that the maximum removal efficiency was reported at pH 5 (100% for Pb2+, 97% for Cu2 + and 55% for Ni2+) with an adsorption capacity of 1.581 mg/g for Cu2+, 1.337 mg/g for Pb2 +, and 0.130 mg/g for Ni2+ ions. These findings confirm previous studies that indicated that Ni2+ ions have less affinity to be adsorbed in the presence of other metal ions and need a selective adsorbent (Noman et al. 2022). Onundi et al. (2011) used a nano-sized composite adsorbent to remove Pb2+, Cu2+, and Ni2+ from a semiconductor effluent. The research claimed that the maximum recovery of heavy metals was 99% for Pb2+, 61% for Cu2+, and 20% for Ni2+ at pH 5, 1 g adsorbent/L and after 1 h of the contact time. The application of a biosorption process using Chlorella sorokiniana to remove gallium from semiconductor wastewater was explored by Li et al. (2018). The research used batch and continuous flow operations and revealed that adsorption capacity was 38.5 mg/g at pH 2.8 and 14.1 mg/g at pH 2.3). These findings indicated the potential of algae-based sorbent as a desirable choice to remove gallium from process tool wastewater. Zou et al. (2007) studied the decontamination of Cu2+ ions, phenol, and ethyl lactate from semiconductor wastewater by UV/TiO2 photocatalytic degradation. The highest degradation of ethyl lactate and phenol was recorded with 0.1 g of TiO2 / L and pH between 3 and 4, while Cu2+ removal occurs as a function of the reduction process through precipitation. Bang et al. (2016) developed a protonated alginate (HA) bead to remove the toxicity of Cu2 + ions from semiconductor wastewater. The assessment of Cu2+ toxicity in the semiconductor wastewater was conducted towards Daphnia magna, which indicated that the Cu2+ exhibited high toxicity in concentrations between 1.97 and 3.37 mg/L. The results revealed that protonation showed high efficiency in reducing Ca2 + cation releases during Cu2+ sorption and improved adsorption capacity from 107 to 189 mg/g. The concentration in the effluent was relational to acute toxicity, where the toxic values grew steeply with 0.1 mg Cu2+/L.
CMP wastewater with high suspended solid concentrations and turbidity because of nano-sized silica (SiO2) particles represents a good resource for recovering and reutilising in treating wastewater (Ohanessian et al. 2020). Qiu et al. (2022) conducted a coagulation-ultrafiltration (UF) operation to eradicate fluoride and silica from semiconductor wastewater using CMP wastewater and polyaluminium chloride/polyacrylamide as coagulants. The results revealed that CaF2 particles reacted with CMP wastewater due to the negatively charged SiO2 particles via electrostatic relation. The results specified that the CMP wastewater rendered a greater 92.89 and 99.72% fluoride removal.
Tounsi et al. (2022) used the electrocoagulation process to remove nitrate and fluoride anions from aqueous solutions as a function of pH, initial concentration, electrode material, current density, and the existence of coexisting ions. The outcome revealed that nitrate removal was 42.83%, while it was 88.28% for fluoride at pH 5.94, with 9.09 mA. cm−2 of current density and 18 mm of distance between electrodes. The removal process takes place as a function of adsorption and reduction processes. Figure 8 presents the most common agriculture wastes that have been used to develop adsorbents from heavy metal removal. Rice husk, wheat straw, sawdust, sludge, and cassava peels are the most familiar adsorbents for heavy metals due to the high contents of lignin and cellulose.
Removal of organic pollutants
Several organic and inorganic pollutants have been reported in the semiconductor wastewater. The ammonium ion is among the contaminants in the semiconductor wastewater, which should be removed before the final discharge of these wastes to prevent eutrophication in the natural water systems. Denitrification, chlorination, and air stripping are the most extensively used technologies for NH4 removal (Chung et al. 2020a, b). The biological process has several limitations due to the high toxicity of metals in the semiconductor wastewaters and, thus, low biodegradability of NH4. The usage of ceramic nanofiltration (NF) membrane to remove organic and inorganic contaminants and NH4 from semiconductor wastewater was studied by Cha et al. (2022a, b). In the study, the NF membrane has a 0.65 nm pore radius with a negative charge at neutral pH. The retention rate was 88.7%. This solution ascribed a sophisticated valence co-ion (SO42−) to a negative surface charge identical to a NF ceramic membrane. Chung et al. (2020a, b) indicated that the application of electrochemical oxidation (EO) to degrade ammonia in semiconductor wastewater is limited by the presence of H2O2, and it is suggested to subject semiconductor wastewater for primary treatment by adsorption to be removed by activated carbon. This process will improve ammonia removal by 2∼3.5. Cha et al. (2022a, b) fabricated graphene oxide (GO) ceramic nanofiltration (NF) membranes using a layer-by-layer (LbL) assembly of GO and polyethyleneimine (PEI) on a ceramic ultrafiltration (CUF) substrate for NH4 retention in semiconductor wastewater. The performance of the GO-CNF membranes was performed with three GO-PEI bilayers, and the removal of NH4 (90.8%) with real semiconductor wastewater was three times higher than the pristine ceramic UF substrate (39.1%).
PFOA is widely used in semiconductor manufacturing, and direct disposal of these wastes is associated with potential toxicity to the environment. This compound is implemented as an emulsifying agent in the polymer synthesis in the photolithography process. PFOA is widely applied in various industries, such as microelectronics surface coating agents and semiconductors. PFOA in the environment has a high resistance to natural degradation and has teratogenicity, carcinogenicity, endocrine disruption activity, and mutagenicity (Kishor et al. 2021). Bao et al. (2022) developed a novel pH-stimulated poly(dimethyl amino) ethyl methacrylate (PDMAEMA) microgel for selective retrieval of PFOA from semiconductor wastewater as a function of adsorption. The investigation showed that the adsorption capacity was 2632 mg PFOA g−1 PDMAEMA in the occurrence of 5% (v/v) isopropyl alcohol (IPA), which is more than that reported by carbon-based materials (50–500 mg g−1) (Yu et al. 2009). PDMAEMA exhibited high efficiency in the adsorption capacity with stability for the pH because the process takes place as a function of electrostatic interaction with hydrophobic interactions, which has less sensitivity for changes in the pH values.
The demographic of semiconductor wastewater treatment plants has been investigated by Serreli et al. (2021). The evaluation was conducted using PSILCA (Product Social Impact Life Cycle Assessment), while 65 social gauges of a wastewater treatment plant were used in this study. The investigation showed that the highest impact is related to tetramethylammonium hydroxide, which has high effects on the local municipal and rises from the indicator influence to environmental load with a health risk. The functional unit exceeded 300,000 intermediate risk hours. Kim et al. (2021) used nano-ozone bubbles to improve the removal efficacy of TMAH from semiconductor wastewater using the ozone/H2O2 process. The study revealed that the H2O2 additives with nano-ozone bubbles improved the degradation of TMAH by 95% at pH 10 and 25 °C within 90 min. Ferella et al. (2021) studied the removal of TMAH based on a pilot scale where three aerobic bioreactors were involved, using a real TMAH solution effluent in a batch operation model. The treatment was carried out for 14 days with a flow rate of 5 L/h. The study revealed that TMAH degradation occurred during the batch study by 80% in the first reactor, while a whole (100%) degradation was recorded in the second and third bioreactors. In the first bioreactor, the microbial density increased by 338 and 58% in the second bioreactor, while amplified by only 1% in the third bioreactor. These might be related to the stage of the degradation process due to the production of enzymes in the secondary metabolic products by the microbes. In the first bioreactor, the microbes multiply while producing more hydrolysis enzymes in the second bioreactor. In the third bioreactor, the microbial growth reached the death stage, where neither multiplication nor enzyme production was produced. Innocenzi et al. (2019) performed an aerobic treatment to remove TMAH, fluorides, nitrates, acetic acid, and phosphates. The aerobic bioreactor was inoculated with an activated sludge from the sewage treatment plant. The study claimed that 99% of TMAH was degraded after seven days, while 99% of fluorides, nitrates, acetic acid, and phosphates were removed by chemical precipitation with lime. In a study conducted on removing TMAH by biological (activated sludge) and chemical (advanced oxidation) processes from microelectronic wastewater, the highest removal of TMAH was > 98%, while it was > 96% for TN (Mehta et al. 2014). In fact, the application of microbial technology for eliminating the pollutants from semiconductor wastes needs a deep understanding of the microbial enzyme, metabolism pathways, and composition of complex microbial communities (Kishor et al. 2021).
Den et al. (2002) tested the pertinency of the photochemical oxidation process (UV/H2O2) combined with the biological fluidised-bed system for the degradation of isopropanol, ethyl lactate, tetramethylammonium hydroxide, phenol, 1-methyl-2-pyrrolidone, and propylene glycol methyl ether acetate in semiconductor wastewater. The degradation process was investigated with a few variables, such as pH, time, and H2O2 dosage. The effectiveness of the treatment was evaluated on the TOC reduction and GCMS analysis. The study demonstrated that the oxidation process exhibited high efficiency in decomposing the chemicals to be efficiently mineralised by aerobic biodegradation with a drop in efficiencies of > 95% in TOC.
Tetramethylammonium (TMA) is used as an etching and photo-developing reagent and thermochemolysis in the production of semiconductors. The compound is very poisonous, causing reproductive toxicity, respiratory failure, and neurotoxicity through skin absorption, inhalation, and ingestion. Liu et al. (2022) used a biological approach to treat TMAC using activated sludge from STP that contains Ochrobactrum spp., Mycobacterium spp., and Arthrobacter species. The treatment process was conducted by manipulating factors such as pH, temperature, and TMAC dosages. The study revealed that degradation occurs through nitrification and denitrification (SND) at pH 3–5, 20–40 °C, and 60% of the nitrogen-containing intermediates containing nitrogen were transformed into gaseous nitrogen (N2).
Zero-discharge concept of microelectronic waste
The semiconductor industries rose between the 1940s and 1960s. Since the 1960s, these industries have started to develop new water and wastewater treatment materials for managing water quality (Fig. 9). Currently, the semiconductor sector constructed the concept of zero discharge for water conservation, recycling, and reuse as part of sustainable strategies, which aim to develop manufacturing processes to maximise resource utilisation, encouraging the semiconductor trade to implement a zero-liquid discharge policy (Laronge and Lisin 2002). Microelectronic effluent polish is performed using an advanced oxidation process that causes the production of effluents and sludge. CMP is a method used in IC chip factories during planarisation, where huge amounts of wastewater and sludge are generated (Lee and Liu 2009).
In recovering metals from semiconductor effluents to a product to achieve the concept of zero discharge, Bayon et al. (2021) investigated the efficiency of homogeneous crystallisation in a fluidised-bed reactor for recovering cobalt, Co, and Cd from semiconductor wastewater. The treatment was conducted at pH of 7.75–8.0 and [PO4−3]/[Co2+] of 2.0. The maximum recovery of Co was 98.8% as a function of crystallisation. EDTA and citrate negatively influenced the metal recovery by 35%. In the mixture of Co and Cu, the maximum granulation of Cu was 95.9% at a pH of 6.50, while for Co, it was 96.8% at a pH of 7.50. Bayon et al. (2019) investigated the potential of fluidised-bed homogeneous crystallisation to recuperate Cu–P granules from semiconductor wastewater. The study revealed that the highest crystallisation and recovery efficiency were 99% for Cu and 96.07% for P, recorded at pH 6.0–6.5. At a hydraulic retention time of 22.5 min [PO43] in / [Cu2+], it was 1.25 with 0.51 kg Cu2+/m2 h and fixed loading of 4.5 mM, the libethenite (Cu2PO4OH) was detected as the main recovered product with a diameter of ∼1 mm. Fluidised-bed crystallisation is an alternative phase-separation technology used for treating semiconductor wastewater and acts with essential principles similar to normal chemical precipitation with crystal growth to produce recovered solids for reuse (Lv et al. 2018).
Struvite (magnesium ammonium phosphate or MgNH4PO4·6H2O) is a phosphate mineral used to eliminate NH4 from semiconductor wastewater via precipitation (Kim et al. 2009). Ryu et al. (2012a, b) studied the potential of struvite as a fertiliser for farming Chinese cabbage compared to commercial fertilisers. The study revealed that Chinese cabbage growth was promoted by applying struvite deposit as fertiliser with 1.6 g of struvite/kg of soil due to the high K, N, P, Ca, and Mg contents. The chemical analysis of the cabbage composition revealed the absence of heavy metals such as Cu, Cd, As, Pb, and Ni ions. In another study, Ryu et al. (2012a, b) assessed the potential of struvite from semiconductor wastewater as a fertiliser for Lactuca sativa cultivation. The study revealed that struvite improved lettuce growth more efficiently than commercial fertilisers. The chemical analysis of lettuce tissue detected the presence of N, P, K, and Mg, with low concentrations of Hg2+, Pb2+, Cr2+, and Ni2+ ions.
Future perspective for treating and recycling microelectronics sludge and wastewater
The industrial production of semiconductor device manufacturing has grown fast in the last few years. The increased production of microelectronics has led to an increase in the amount of sludge-waste generated, which has become a major environmental concern. Recycling effectively manages electronic waste; the mechanism studies on sludge resource recovery are still in their infancy. In the field of sludge reclamation and reuse technologies, increasing attention is being paid to producing clean sludge that has less volume and can be safely reused. Development in this area has been slower than in the field of wastewater treatment. Microelectronics sludge (MES) is produced while treating microelectronics wastewater; the MES contains heavy metals such as copper and other ions such as calcium and magnesium. The current treatment process includes dewatering and sludge thickening. These methods are insufficient to achieve the concept of zero discharge. Semiconductor wastewater contains high ammonium ions from the utilisation of raw substances during the manufacturing process. Denitrification, chlorination, and air stripping are the most used technologies for NH4 removal but have several limitations due to the high toxicity of metals. On the other hand, the high contents of the metal hydroxides in the MES improve its quality as an adsorbent because of its unique structure in terms of high surface area, lamellar structure, and high ion-exchange capacity. MES with Mg2+, Ca2+, Al3+, or Fe3+ contents might improve the adsorption efficiency via anion exchange. The development of activated carbon, mesoporous nanomaterials, metal (hydr)oxides, and composite materials from inorganic sludge have been reported in the literature. These materials generally require high preparation costs and a complex process of preparation. It is desirable to use low-cost, high-metal adsorbents. The reuse of MES not only reduces solid waste in wastewater treatment but also produces cheap adsorbents to remove heavy metal ions.
Lee and Liu (2009) used dried and pulverised sludge recovered from semiconductor industries to replace Portland cement (5–20 wt%) in cement mortar. The results revealed that the mortar compressive strength improved by 25–35% with 10% of the substitution after 90 days of the curing period. The toxicity characteristics leaching procedure (TCLP) analysis of the cement mortar has not detected heavy metals released. The increase in mortar strength might be related to the presence of large hydration compounds generated from the use of sludge from semiconductor industries, which were subjected to deflocculates in acidic and alkaline mediums in nanoparticles lesser than 100 nm. Liu et al. (2011) utilised the mixtures of sludge with CaF2 from semiconductor wastewater to produce glass ceramics. CaF2 is generated by using hydrofluoric acid to clean wafers in semiconductor industries. In the study, the CaF2 sludge was mixed with a conditioner to reduce the melting points of the calcium oxide- Si dioxide-aluminium oxide glass ceramics. The study indicated that the melting temperature dropped from 1378 to 1163 °C with a 6:4 ratio (sludge-waste glass). The reduction in the melting point was associated with minimising heavy metal leachate from glass ceramics.
A primary study has been conducted on the removal of pollutants from microelectronics industry wastewater. The study intended to synthesise a nanoporous microelectronics sludge graphite oxide adsorbent (NMSAGO) to remove ammonium from microelectronic wastewater (Fig. 10). The main target for dealing with MES and wastewater generated from microelectronic wastewater is to recycle or generate a new product from the sludge to remove pollutants such as TMAH, ammonium, and heavy metals, which are the major pollutants in microelectronic wastewater. The preparation of adsorbent and NMSAGO represents good examples of circular economy and minimises the cost associated with using expensive technologies such as membrane technology. The preparation of NMSAGO from MES is in line with two concepts, including zero discharge and circular economy and engaging with the microelectronic industry to study the applicability of MES for treating MEW before being released to the environment, to refine policy and improve regulatory control in wastewater management. Moreover, the life cycle assessment (LCA) and techno-economic analysis (TEA) of NMSAGO should be investigated to provide more information on the applicability of the NMSAGO as a valid product without impact on the environment in line with the Safety Materials and Nano Products Risk Benchmark adopted by several countries.
Ammonium is among the pollutants easily detected in microelectronic wastewater but represents the main challenge in the recycling of microelectronic wastewater. The recovery of ammonium from microelectronic wastewater has several limitations, including but not limited to other pollutants, such as heavy metals, which compete with ammonium and have an affinity to be adsorbed on the adsorbent. Therefore, a selective adsorbent based on the chemical composition and specific surface properties of the adsorbent might provide high removal efficiency for ammonium from microelectronics wastewater. Theoretically, ammonium can be adsorbed on a selective adsorbent with several abundant functional groups. MSE with a high content of metal hydroxides improves the properties of the adsorption process. The unique structure in terms of high surface area, lamellar structure, and high ion-exchange capacity in the MSE might contribute effectively to recovering ammonium. Moreover, the preparation of a MSE nanoporous absorbent composite with graphite oxide might enhance the chemical composition and surface properties with a high potential to remove ammonium from nutrient-rich wastewater.
In the optimisation of ammonium absorption, the main operational parameters/factors are contact time, pH value, adsorbent dosage, and initial concentration of ammonium, as shown in Table 2. Investigation of the effect of contact time on ammonium removal showed that efficiency increases with increasing contact time. It should be noted that the adsorption rate increases significantly at the initial contact time. This high initial removal rate can be attributed to the concentration gradient created between the ammonium molecules and the availability of the adsorption sites on the absorbent (Fathi et al. 2022). The pH of the solution has an important effect on the ammonium ions and surface characteristics of the adsorbents. Variation of pH in the solution can cause protonation and deprotonation of functional groups on the surface of clays by the adsorption of H + and OH-. Ammonium adsorption increases as pH increases (Hashemi et al. 2021). The maximum adsorption of ammonium was obtained at high pH. This could be because the weak positive charge of nonpolar compounds is at its maximum at low pH, and this low surface charge decreases rapidly with increasing pH (Fathi et al. 2022). In fact, negative charges increase with increasing pH and lead to an increase in adsorption capacity (Hashemi et al. 2021).
Conclusion
The semiconductor industry is among the microelectronics sectors that contribute effectively to environmental pollution by producing huge amounts of wastewater containing organic and inorganic pollutants. Taiwan is one of the leading countries in the semiconductor industry. Si residues are among the metals in the microelectronics wastewater subjected to recovery and recycling into new products. Ultrafiltration, reverse osmosis (RO), and electrolysis exhibit high efficiency in producing ultrapure water. At the same time, chemical treatment methods such as the oxidation process have more efficiency in the degradation of organic compounds compared to the biological process, which has several limitations due to the high toxicity of the microelectronics wastewater with different contaminants. Microelectronics sludge is the main challenge in the zero-discharge concept due to the unavailable, more efficient technologies for recycling these wastes, which are disposed of into the environment. Based on the review process, it could appear that:
-
(a)
More progressive types of machinery are essential to improve the quality of the semiconductor wastewater before being recycled into ultrapure water
-
(b)
In the field of microelectronics sludge, further studies are required to provide and develop a treatment technology to achieve the zero-discharge concept.
Data availability
Not applicable.
Abbreviations
- EPR:
-
Extended producer responsibility
- WWTP:
-
Wastewater treatment plant
- TMAH:
-
Tetramethylammonium hydroxide
- CMPW:
-
Chemical mechanical polishing wastewater
- CMPP:
-
Chemical mechanical polishing process
- PFOA:
-
Perfluorooctanoic acid
- UPW:
-
Ultrapure water
- AOP:
-
Advanced oxidation process
- UFRO:
-
Ultrafiltration reverse osmosis
- RODI:
-
Reverse osmosis/deionisation
- UF:
-
Ultrafiltration
- HRT:
-
Hydraulic retention time
- EO:
-
Electrochemical oxidation
- GO:
-
Graphene oxide
- PEI:
-
Polyethyleneimine
- CUF:
-
Ceramic ultrafiltration
- TMA:
-
Tetramethylammonium
- MES:
-
Microelectronics sludge
- TCLP:
-
Toxicity characteristics leaching procedure
References
Adam MR, Othman MHD, Hubadillah SK, Abd Aziz MH, Jamalludin MR (2023) Application of natural zeolite clinoptilolite for the removal of ammonia in wastewater. Materials Today: Proceedings
An SA, Lee J, Sim J, Park CG, Lee JS, Rho H, Park KD, Kim HS, Woo YC (2022) Evaluation of the advanced oxidation process integrated with microfiltration for reverse osmosis to treat semiconductor wastewater. Process Saf Environ Prot 162:1057–1066
Bang S, Choi JW, Cho K, Chung C, Kang H, Hong SW (2016) Simultaneous reduction of copper and toxicity in semiconductor wastewater using protonated alginate beads. Chem Eng J 288:525–531
Bao Y, Gupta N, Chuah CY, Liang YN, Hu CP, Hu X (2022) Highly selective recovery of perfluorooctanoic acid from semiconductor wastewater via adsorption on pH-stimulated poly (dimethyl amino) ethyl methacrylate microgels. Sep Purif Technol 287:120479
Bayon LLE, Ballesteros FC Jr, Choi AES, Garcia-Segura S, Lu MC (2021) Remediation of cobalt from semiconductor wastewater in the frame of fluidised-bed homogeneous granulation process. J Environ Chem Eng 9(5):105936
Bayon LLE, Ballesteros FC Jr, Garcia-Segura S, Lu MC (2019) Water reuse nexus with resource recovery: On the fluidised-bed homogeneous crystallisation of copper and phosphate from semiconductor wastewater. J Clean Prod 236:117705
Belongia BM, Haworth PD, Baygents JC, Raghavan S (1999) Treatment of alumina and silica chemical mechanical polishing waste by electrodecantation and electrocoagulation. J Electrochem Soc 146(11):4124
Cha M, Boo C, Park C (2022a) Simultaneous retention of organic and inorganic contaminants by a ceramic nanofiltration membrane for the treatment of semiconductor wastewater. Process Saf Environ Prot 159:525–533
Cha M, Boo C, Song IH, Park C (2022b) Investigating the potential of ammonium retention by graphene oxide ceramic nanofiltration membranes for the treatment of semiconductor wastewater. Chemosphere 286:131745
Chen Z, Wu G, Wu Y, Wu Q, Shi Q, Ngo HH, Saucedo OAV, Hu HY (2020) Water eco-nexus cycle system (WaterEcoNet) as a key solution for water shortage and water environment problems in urban areas. Water Cycle 1:71–77
Choi J, Dorji P, Shon HK, Hong S (2019) Applications of capacitive deionisation: desalination, softening, selective removal, and energy efficiency. Desalination 449:118–130
Chung J, Choi J, Chung S (2020a) Pilot study of specific microbe immobilisation cells (SMICs) technology in removing tetramethyl ammonium hydroxide for reuse of low-strength electronics wastewater. J Hazard Mater 384:120829
Chung S, Chung J, Chung C (2020b) Enhanced electrochemical oxidation process with hydrogen peroxide pretreatment for removal of high strength ammonia from semiconductor wastewater. J Water Process Eng 37:101425
Chung J, Fleege D, Ong SK, Lee YW (2014) Organic semiconductor wastewater treatment using a four-stage Bardenpho with membrane system. Environ Technol 35(22):2837–2845
Cieślik B, Konieczka P (2017) A review of phosphorus recovery methods at various steps of wastewater treatment and sewage sludge management. The concept of “no solid waste generation” and analytical methods. J Clean Prod 142:1728–1740
Dabwan HA, Jaafar FRBC (2018) Towards zero emission concepts: high content colloidal silica removal by using paper sludge ash as inorganic coagulant. Mater Today Proc 5(10):21559–21565
Den W, Ko FH, Huang TY (2002) Treatment of organic wastewater discharged from semiconductor manufacturing process by ultraviolet/hydrogen peroxide and biodegradation. IEEE Trans Semicond Manuf 15(4):540–551
Ding Y, Sartaj M (2015) Statistical analysis and optimisation of ammonia removal from aqueous solution by zeolite using factorial design and response surface methodology. J Environ Chem Eng 3(2):807–814
Drouiche N, Ghaffour N, Lounici H, Mameri M (2007) Electrocoagulation of chemical mechanical polishing wastewater. Desalination 214(1–3):31–37
Eng CY, Yan D, Withanage N, Liang Q, Zhou Y (2019) Wastewater treatment and recycle from a semiconductor industry: a demo-plant study. Water Practice Technol 14(2):371–379
Fatehah MO, Hossain MS, Teng TT (2013) Comparative study on natural and commercial coagulants: treatment of semiconductor wastewater in sludge production and removal of heavy metals. Int J Sci Mod Eng 1:1-8
Fathi R, Mohammadi P, Hosseini SA, Yosefvand F, Norouzi H (2022) Modeling of ammonia removal from wastewater using air stripping/modified clinoptilolite: reusability, optimisation, isotherm, kinetic, and equilibrium studies. Int J Environ Sci Technol 19(4):2493–2514
Ferella F, Innocenzi V, Moretti G, Zueva SB, Pellegrini M, De Michelis I, Ippolito NM, Del Gallo M, Prisciandaro M, Vegliò F (2021) Water reuse in a circular economy perspective in a microelectronics industry through biological effluents treatments. J Clean Prod 320:128820
Forti V, Balde CP, Kuehr R, Bel G (2020) The Global E-waste Monitor 2020: quantities, flows and the circular economy potential. United Nations University (UNU)/United Nations Institute for Training and Research (UNITAR) – co-hosted SCYCLE Programme, International Telecommunication Union (ITU) & International Solid Waste Association (ISWA), Bonn/Geneva/Rotterdam
Haddaway NR, Page MJ, Pritchard CC, McGuinness LA (2022) PRISMA2020: an R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev 18(2):e1230
Hashemi H, Bahrami S, Emadi Z, Shariatipor H, Nozari M (2021) Optimisation of ammonium adsorption from landfill leachate using montmorillonite/hematite nanocomposite: response surface method based on central composite design. Desalin Water Treat 232:39–54
Hsu SC, Hsieh HL, Chen CP, Tseng CM, Huang SC, Huang CH, Huang YT, Radashevsky V, Lin SH (2011) Tungsten and other heavy metal contamination in aquatic environments receiving wastewater from semiconductor manufacturing. J Hazard Mater 189(1–2):193–202
Hsu LC, Huang CY, Chuang YH, Chen HW, Chan YT, Teah HY, Chen TY, Chang CF, Liu YT, Tzou YM (2016) Accumulation of heavy metals and trace elements in fluvial sediments received effluents from traditional and semiconductor industries. Sci Rep 6(1):1–12
Innocenzi V, Zueva SB, Ippolito NM, Ferella F, Prisciandaro M, Vegliò F (2022) A review of the existing and emerging technologies for wastewaters containing tetramethyl ammonium hydroxide (TMAH) and waste management systems in micro-chip microelectronic industries. Chemosphere 307:135913
Innocenzi V, Zueva S, Prisciandaro M, De Michelis I, Di Renzo A, Di Celso GM, Vegliò F (2019) Treatment of TMAH solutions from the microelectronics industry: a combined process scheme. J Water Process Eng 31:100780
Kemp S (2020) Digital use around the world in July 2020. Retrieved from Wearesocial.com: https:// wearesocial.com/blog/2020/07/digital-use-around-the-world-in-july-2020
Kim TK, Kim T, Lee I, Choi K, Zoh KD (2021) Removal of tetramethylammonium hydroxide (TMAH) in semiconductor wastewater using the nano-ozone H2O2 process. J Hazard Mater 409:123759
Kim D, Kim J, Ryu HD, Lee SI (2009) Effect of mixing on spontaneous struvite precipitation from semiconductor wastewater. Biores Technol 100(1):74–78
Kishor R, Bharagava RN, Ferreira LFR, Bilal M, Purchase D (2021) Molecular techniques used to identify perfluorooctanoic acid degrading microbes and their application in a wastewater treatment reactor/plant. Wastewater treatment reactors. Elsevier, pp 253–271
Lacson CFZ, Lu MC, Huang YH (2021) Fluoride-rich wastewater treatment by ballast-assisted precipitation with the selection of precipitants and discarded or recovered materials as ballast. J Environ Chem Eng 9(4):105713
Laronge TM, Lisin MA (2002) Water conservation and material issues in microelectronics production. Ultrapure Water 19(1):32–39
Lee H, Jin Y, Hong S (2016) Recent transitions in ultrapure water (UPW) technology: rising role of reverse osmosis (RO). Desalination 399:185–197
Lee TC, Liu FJ (2009) Recovery of hazardous semiconductor-industry sludge as a useful resource. J Hazard Mater 165(1–3):359–365
Li M, Shadman F, Ogden KL (2018) Algae-based sorbents for removal of gallium from semiconductor manufacturing wastewater. Clean Technol Environ Policy 20(4):899–907
Liu YC, Hu YC, Chu IM, Wei YH, Tsai SL (2022) Biodegradation of tetramethylammonium chloride wastewater and inorganic nitrogen removal by a mixed culture. J Environ Chem Eng 10(1):106931
Liu WT, Li KC (2011) Application of reutilization technology to calcium fluoride sludge from semiconductor manufacturers. J Air Waste Manag Assoc 61(1):85–91
Liu CC, Liu JC (2016) Coupled precipitation-ultrafiltration for treatment of high fluoride-content wastewater. J Taiwan Inst Chem Eng 58:259–263
Lv XM, Li J, Chen H, Tang HJ (2018) Copper wastewater treatment with high concentration in a two-stage crystallisation-based combined process. Environ Technol 39(18):2346–2352
Mao G, Han Y, Liu X, Crittenden J, Huang N, Ahmad UM (2022) Technology status and trends of industrial wastewater treatment: a patent analysis. Chemosphere 288:132483
Mehta S, Chowdhury N, Horner D, Lau A, Schilling B (2014) A combined biological and advanced oxidation process for the treatment of wastewaters from the microelectronics industry. In: WEFTEC 2014, Water Environment Federation
Melnik LA, Krysenko DA (2019) Ultrapure water: properties, production, and use. J Water Chem Technol 41(3):143–150
Mirahsani A, Giorgi JB, Sartaj M (2020) Ammonia removal from aqueous solution by sodium functionalised graphene oxide: isotherm, kinetics, and thermodynamics. Desalin Water Treat 178:143–154
Moradi M, Fazlzadehdavil M, Pirsaheb M, Mansouri Y, Khosravi T, Sharafi K (2016) Response surface methodology (RSM) and its application for optimisation of ammonium ions removal from aqueous solutions by pumice as a natural and low cost adsorbent. Arch Environ Prot 42(2):33–43
Mori IC, Arias-Barreiro CR, Koutsaftis A, Ogo A, Kawano T, Yoshizuka K, Inayat-Hussain SH, Aoyama I (2015) Toxicity of tetramethylammonium hydroxide to aquatic organisms and its synergistic action with potassium iodide. Chemosphere 120:299–304
Ning RY (2003) Discussion of silica speciation, fouling, control and maximum reduction. Desalination 151(1):67–73
Noman EA, Al-Gheethi A, Al-Sahari M, Mohamed RMSR, Crane R, Ab Aziz NA, Govarthanan M (2022) Challenges and opportunities in the application of bioinspired engineered nanomaterials for the recovery of metal ions from mining industry wastewater. Chemosphere 136165
Ohanessian K, Monnot M, Moulin P, Ferrasse JH, Barca C, Soric A, Boutin O (2020) Dead-end and crossflow ultrafiltration process modelling: application on chemical mechanical polishing wastewaters. Chem Eng Res Des 158:164–176
Omar F, Sohrab H, Tjoon Tow T (2013) Semiconductor wastewater treatment using tapioca starch as a natural coagulant. J Water Resour Prot 5:9
Onundi YB, Mamun AA, Al Khatib MF, Ahmed YM (2010) Adsorption of copper, nickel and lead ions from synthetic semiconductor industrial wastewater by palm shell activated carbon. Int J Environ Sci Technol 7(4):751–758
Onundi YB, Mamun AA, Al Khatib MF, Al Saadi MA, Suleyman AM (2011) Heavy metals removal from synthetic wastewater by a novel nano-size composite adsorbent. Int J Environ Sci Technol 8(4):799–806
Palmer M, Hatley H (2018) The role of surfactants in wastewater treatment: impact, removal and future techniques: a critical review. Water Res 147:60–72
Post GB, Cohn PD, Cooper KR (2012) Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: a critical review of recent literature. Environ Res 116:93–117
Qiu Y, Ren LF, Xia L, Shao J, Zhao Y, Van der Bruggen B (2022) Investigation of fluoride and silica removal from semiconductor wastewaters with a clean coagulation-ultrafiltration process. Chem Eng J 438:135562
Rajesh R, Kanakadhurga D, Prabaharan N (2022) Electronic waste: a critical assessment on the unimaginable growing pollutant, legislations and environmental impacts. Environ Chall 7:100507
Ryu HD, Lim CS, Kang MK, Lee SI (2012a) Evaluation of struvite obtained from semiconductor wastewater as a fertiliser in cultivating Chinese cabbage. J Hazard Mater 221:248–255
Ryu HD, Lim CS, Kim YK, Kim KY, Lee SI (2012b) Recovery of struvite obtained from semiconductor wastewater and reuse as a slow-release fertiliser. Environ Eng Sci 29(6):540–548
Schischke K, Stutz M, Ruelle JP, Griese H, Reichl H (2001) Life cycle inventory analysis and identification of environmentally significant aspects in semiconductor manufacturing. In: Proceedings of the 2001 IEEE international symposium on electronics and the environment. 2001 IEEE ISEE (Cat. No. 01CH37190), IEEE, pp 145–150
Serreli M, Petti L, Raggi A, Simboli A, Iuliano G (2021) Social life cycle assessment of an innovative industrial wastewater treatment plant. Int J Life Cycle Assess 26(9):1878–1899
Teow YH, Chiah YH, Ho KC, Mahmoudi E (2022) Treatment of semiconductor-industry wastewater with the application of ceramic membrane and polymeric membrane. J Clean Prod 337:130569
Tounsi H, Chaabane T, Omine K, Sivasankar V, Sano H, Hecini M, Darchen A (2022) Electrocoagulation in the dual application on the simultaneous removal of fluoride and nitrate anions through respective adsorption/reduction processes and modelling of continuous process. J Water Process Eng 46:102584
Trend Force (2022) Localisation of chip manufacturing rising. Taiwan to control 48% of global foundry capacity in 2022. https://www.trendforce.com/presscenter/news/20220425-11204.html
Wang Q, Luo L, Huang N, Wang W, Rong Y, Wang Z, Yuan Y, Xu A, Xiong J, Wu Q, Hu H (2022) Evolution of low molecular weight organic compounds during ultrapure water production process: a pilot-scale study. Sci Total Environ 830:154713
Wu N, Wyart Y, Rose J, Angeletti B, Moulin P (2012) Influence of wastewater treatment from microelectronics industry on concentrations and partitioning of elements in a river. In: International conference on ecology, waste recycling, and environment, Macao
Xiao Y, Chen T, Hu Y, Wang D, Han Y, Lin Y, Wang X (2014) Advanced treatment of semiconductor wastewater by combined MBR–RO technology. Desalination 336:168–178
Yang BM, Huang CJ, Lai WL, Chang CC, Kao CM (2012) Development of a three-stage system for the treatment and reclamation of wastewater containing nano-scale particles. Desalination 284:182–190
Yu Q, Zhang R, Deng S, Huang J, Yu G (2009) Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: Kinetic and isotherm study. Water Res 43(4):1150–1158
Zou SW, How CW, Chen JP (2007) Photocatalytic treatment of wastewater contaminated with organic waste and copper ions from the semiconductor industry. Ind Eng Chem Res 46(20):6566–6571
Acknowledgements
This research was supported by Ministry of Higher Education (MOHE) through Fundamental Research Grant Scheme (FRGS/1/2023/WAB02/UTHM/03/2).
Funding
The authors did not receive support from any organisation for the submitted work.
Author information
Authors and Affiliations
Contributions
EN: methodology investigation and manuscript draft. AAG: conceptualization, supervision, writing–review & editing. MAS: writing–review & editing. GY: writing–review and editing. RMSRM: writing–review and editing. CFS: writing–review & editing. HHTN: writing–review & editing. DVNV: writing–review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that there are no known conflicts of interest associated with this publication and that there has been no significant financial support for this work that could have influenced its outcome.
Ethical approval
We confirmed that the current work is original for the authors and has not been published or under review in any journal.
Consent to participate
All authors have read and contributed to this manuscript and agree on the submission.
Consent to publish
All authors have been read and contributed to this manuscript and agree for the publications.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Noman, E.A., Ali Al-Gheethi, A., Al-Sahari, M. et al. An insight into microelectronics industry wastewater treatment, current challenges, and future perspectives: a critical review. Appl Water Sci 14, 64 (2024). https://doi.org/10.1007/s13201-024-02104-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02104-7