Abstract
Paper presents the results of investigations on sorption capacity of selected carcinogenic polycyclic aromatic hydrocarbons (PAHs) on microplastics fibers. Accumulation of organic micropollutants such as PAHs is indicated as one of the important problems in the case of microplastics present in surface water, including sea water. Concentrations of the micropollutants can reach even several mg/kg of microplastics. An unrecognized problem are sorption capacities of microfibers towards PAHs. Recent research works indicate that microfibers are commonly present in sewage sludge. Content of the fibers in this waste material can reach over 23000 particles per 1 kg of sludge dry matter. The fibers mainly come from cloth washing. It is obvious that the adsorption capacity of the fibers can affect the concentration of PAHs in sewage sludge. The aim of the study was to evaluate sorption capacities of polyester fibers towards PAHs. Adsorption of PAHs were provided under static conditions. After 24 h of adsorption 1255 μg/g of carcinogenic PAHs was adsorbed onto the polyster fibers. Amount of 6-ring PAHs was equal to 154 μg/g, whereas 5- i 4- ring ones, 562 and 539 μg/g, respectively. The results have confirmed that hydrophobic PAHs can be adsorbed onto polystyrene fibers and as a result cumulate in sewage sludge.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Synthetic fibers are one of the groups of microplastic pollutants (small plastic particles of sizes in the range of 0.001 to 5 mm) present in wastewater (Kershaw 2015). Microfibers refer to the length to diameter ratio greater than 100 (Palacios-Mateo et al. 2021). Fibers in wastewater originate from washing of synthetic textiles (Hann et al. 2018). It was proved that synthetic clothes contribute to about 35% of the global release of primary microplastics to the world oceans (Falco et al. 2019). It is estimated that a city of 100,000 PE can sent from 5 to more than 60 kg of synthetic microfibers daily to the surface waters. It is equal to even 22 tones per year (Harrison 2018). Microfibers released into the wastewater can vary a lot depending on the type of fabric, cutting method, number o washing cycles. Release of microfibers to influent can reach 72,000 microfibers per gram of textile per wash. But it can be also significantly smaller (in the range of 200 microfibers per gram per wash). The median length of the released fibers was estimated as 165–841 µm (Cai et al. 2020). Effluents from washing machines can contain 100–300 fibers per one liter (Jemec et al. 2016). Most of (up to 99%) microfibers present in wastewater are released from liquid phase and retained in sludge. With this waste material, they can be discharged into the environment. One of the most frequently found chemical groups of microfibers are the polyester ones (Kärkkäinen and Sillanpää 2021). Polyester fibers are produced from polyethylene tetraphtalate (PET), polymer which not exists in nature (Grishanov 2011). The structure of the polyester fibers is a mixture of crystalline (of high density) and non-crystaline regions. They are characterized by low moisture absorption, and ability to accumulate electrostatic charges on the surface. They show a tendency to form entangled bundles of fibers. They are resistant to dilute acids, alkali and organic solvents. If concentrated reagents are used they can damage polyester fibers (Grishanov 2011).
Polyester microfibers can be toxic for living organisms, e.g. Daphnia magna were found to ingest polyester fibers and as a consequence increase in their mortality occurred (Jemec et al. 2016). Microfibers have rather a high surface area and because of this, they are expected to accumulate other environmental pollutants (Palacios-Mateo et al. 2021; Beverley et al. 2019). Research works concerning adsorption of the organic and inorganic micropollutants on the surface of microplastics are still missing. Some studies were made concerning polycyclic aromatic hydrocarbons (PAHs) adsorption on microplastics. They have indicated that sorption of PAHs on microplastic fibers was hydrophobicity dependent (Li et al. 2020a; Qi et al. 2020). The authors have also stated that sorption of PAHs on the microplastic particles was mainly based on partitioning. Among other processes involved in PAHs bonding onto the surface of microplastics surface sorption (hydrogen bonding, π-πinteraction, electrostatic interaction, van der Waals force), as well as pore-filling are pointed out (Wang et al. 1827). Kind of microplastics is also an important factor in organic micropollutants adsorption, e.g. Valzeboer et al. (2014) have stated that polychlorinated biphenyls (PCBs) were better adsorbable to polystyrene than polyethylene. It was explained by the authors by the stronger aromaticity of polystyrene than polyethylene. Desorption was also observed and it was mainly dominated by film diffusion (Li et al. 2020a). It was also stated by other authors that on the surface of microplastics bacteria film can be formed, and this film can affect adsorption, desorption behavior of such micropollutants as PAHs (José and Jordao xxxx).
Research works on various micropollutants adsorption on microplastic fibers are still missing. The present study was aimed at the evaluation of sorption capacity of polyester microfibers towards selected polycyclic aromatic hydrocarbons (PAHs) from treated coke plant wastewater.
Materials and methods
Materials
Effluents from industrial wastewater treatment plants were used as a matrix for the experiment. Biological treatment involved activated sludge technology with biological nitrogen removal. Effluents contained PAHs which did not undergo removal during coke-plant wastewater treatment. The samples were taken once, as we were interested in initial studies on adsorption capacity of polyester fibers.
Experimental procedure
Initial PAHs concentration was analyzed in treated coke plant wastewater. This type of wastewater is released to the sanitary networks and inflow to municipal wastewater treatment plants. Despite biological treatment (in our case activated sludge treatment including nitrification, which means long solid retention time, above 10 days). 300 mL of wastewater was used for the experiment, to each 0.5 g/L of polyester microfibers has been added. pH of wastewater was 7.2. The samples were shaken on shaker with horizontal motion for 24 h at a constant temperature of 20 °C. Concentration of 4-, 5- and 6-ring PAHs in wastewater was analyzed after 1, 2, 16, 18, 20, and 24 h after polyester microfibers were added. Percent removal of individual PAH compounds was calculated as the difference between initial PAHs concentration and concentration after a defined time. Blank sample was prepared to catch the changes in PAHs concentration in wastewater not caused by adsorption but through the other processes. Calculated amount of removed PAHs was compared to the mass of microfibers and expressed as μg per one g of polyester fibers.
Analytical methods of PAHs
Liquid–liquid method was used for selected PAH extraction from wastewater samples. Cyclohexane, dichloromethane (v/v, 20:5:1) were used as extracting agents. The samples were shaken for 1 h on a shaker with horizontal motion at constant amplitude. The extracts were separated from wastewater and simultaneously cleaned by using liquid–solid extraction method. Silica gel columns were used. Cleaned extracts were concentrated to 2 mL under a nitrogen stream. PAHs were determined quantitatively and qualitatively using gas chromatography coupled with mass spectrometer (GC–MS). The analysis was performed on a column DB-5 ms, length of 30 m and a diameter of 0.25 mm. Helium was used as a carrier gas (flow rate 1.08 mL/min). The time of analysis was equal to 50 min. The flow rate was 1.5 mL/min. Eight PAHs (benzo(a)anthracene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, dibenzo(ah)anthracene, indeno(123 cd)pyrene, and benzo(ghi)perylene) showing carcinogenic properties were analyzed.
Results and discussion
The initial concentration of carcinogenic compounds in coke plant wastewater used during the study reached 1904 μg/L. As a result of adsorption process the total concentration of PAHs in wastewater significantly decreased as a result of adsorption process. Amounts of individual PAH compounds in wastewater as a function of time are presented in Fig. 1–8.
In the case of benzo(a)anthracene (Fig. 1) maximum amount of adsorbed compound was equal to 423 μg/g and it was achieved after 24 h. However, after 2 h 190 μg/g of benzo(a)anthracene was adsorbed on microfibers, which is equivalent to about 45% of maximum value. There were no directly proportional relationship between the amount of benzo(a)anthracene and time. The characteristics of the sorption behavior could be connected with the fact that the amounts of benzo(a)anthracene adsorbed were a sum of the compound adsorbed after each period of time. We could only observe the changes of concentrations adsorbed on the plastic fibers, but the data are not sufficient to explain what happened during 14 h period of break. It needs more detailed research work.
In contrast to benzo(a)anthracene in the case of chrysene (Fig. 2) increase of amount of the pollutant adsorbed on microfibers was proportional in time. At the beginning of the experiment less than 11% (13 μg/g) of the chrysene was adsorbed onto the microfibers. Maximum amount of chrysene adsorbed on microfibers was equal to 116 μg/g after 24 h.
Similar patterns were observed in the case of benzo(b)fluoranthene and benzo(k)fluoranthene—Figs. 3 and 4. In the case of benzo(b)fluoranthene maximum amount adsorbed on the microfibers was equal to 179 μg/g and in the case of the benzo(k)fluoranthene 178 μg/g. During the first 2 h, about 22% of maximum amount of these compounds were adsorbed onto microfibers.
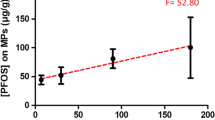
Figure 5 shows changes in benzo(a)pyrene amounts adsorbed onto microfibers during the experiment. The pattern of adsorption was in this case similar to most hydrocarbons analysed during the study. After 2 h 22% of maximum amount was adsorber by the microfibers. Maximum amount adsorber during the 24-h experiment was equal to 142 μg/g.
Amounts of dibenzo(ah)antracene adsorber on microfibers are shown in Fig. 6. Maximum amount adsorber on microfibers was equal to 63 μg/g. After first 2 h, about 19% of this compound has been adsorbed onto microfibers.
Amounts of 6-ring PAHs adsorbed on the microfibers are presented in Figs. 7 and 8. Based on their logKow values these compounds (> 7), they exhibit the largest affinity to solids. The maximum amounts of these compounds adsorbed on microfibers (after 24 h) 67 and 87 μg/g for indeno(1,2,3,c,d)pyrene and benzo(ghi)perylene, respectively. During first 2 h of adsorption 26% of indeno(1,2,3,c,d)pyrene maximum amount was adsorbed, whereas in the case of benzo(ghi)pyrene it was 37%. It means that 6-ring PAHs were adsorbed more rapidly onto microfibers than lower ring PAHs.
In total, 1255 μg/g of carcinogenic PAHs were adsorbed onto polystyrene microfibers. It confirms the thesis that during wastewater treatment and sludge processing these compounds can be adsorbed onto microfibers. Based on the initial and final amount of PAHs in wastewater sample, it was stated that 89.5% of total PAHs were adsorbed on the microfibers during the experiment. The percent amounts of PAHs adsorbed on the microfibers are presented in Fig. 9. In all cases, despite of chrysene, more than 80% of individual compounds present in wastewater have been adsorbed on microfibers, however, no linear correlation can be found between the number of rings in PAHs particle and removal efficiency. Absorption capacity of carcinogenic PAHs (1255 μg/g) was high compared to the results obtained by other authors. Most studies concerning PAHs adsorption on plastic debris indicate that sorption capacity of PAHs to microplastics is high (Sørensen et al. 2020; Lee et al. 2014). PAHs removal efficiency from coke wastewater on mineral sorbent was analyzed in previous research of authors. The percentage removal of 16 hydrocarbons did not exceed 53% on average. The efficiency of removal was highest in the case of 5-ring of hydrocarbons (65%) (Smol and Włodarczyk-Makuła 2017; Włodarczyk-Makuła and Wiśniowska 2018). The studies by other authors have shown that sorption capacity of PAHs on the microfibers depends on temperature. Adsorption was faster as the temperature was higher (the results have concerned sea water) (Sørensen et al. 2020). It is, however, known that PAHs amounts adsorbed onto microplastics can be orders of magnitude greater than surrounding waters (Ziccardi et al. 2016). Salinity of water can affect PAHs sorption behavior in water, e.g. compared to fresh water high salination of seawater can enhance dipole–dipole and dipole-induced dipole interactions between PAHs and microplastics, which can make hydrogen bonds form easily (Li et al. 2020b).
There are no evident proof that polystyrene fibers or other plastic debris are the routes of the exposure of PAHs to water organisms (Ziccardi et al. 2016). The results of toxicity assays indicate that microplastics can induce a slight cellular toxicity in a short time (up to 28 days) period. Bioaccumulation of selected PAHs adsorption onto the surface of microplastics is still not recognized and risk not evaluated (Pittura et al. 2018). No detailed studies on PAHs adsorption from wastewater have been performed.
Conclusions
It can be concluded that:
-
1.
PAHs were effectively adsorbed onto the surface of microfibers present in wastewater.
-
2.
Removal efficiencies of PAHs were in most cases higher than 80%, except for chrysene with a removal rate equal to 69%.
-
3.
PAHs with higher logKow values were more effectively removed as a result of adsorption, but no linear correlation has been observed between logKow and percent removal of individual compounds.
-
4.
Total amount of adsorbed PAHs was equal to 1255 μg/g which was high value compared to reported in papers concerning adsorption of PAHs from fresh and seawater.
-
5.
Further detailed studies on adsorption mechanisms are necessary.
References
Beverley H, Laitala K, Klepp IG (2019) Microfibers from apparel and home textiles: prospects for including microplastics in environmental sustainability assessment. Sci Total Environ 652:483–494
Cai Y, Yang T, Mitrano DM, Heuberger M, Hufenus R, Nowack B (2020) Systematic study of microplastic fiber release from 12 different polyster textiles during washing. Environ Sci Technol 54(8):4847–4855
De Falco F, Di Pace E, Cocca M (2019) Avella M., The contribution of washing processes of synthetic clothes to microplastic pollution, Sci Rep 9: 6633.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6488573/
Grishanov S (2011) Structure and properties of textile materials, In: Handbook of Textile and Industrial Dyeing, https://www.sciencedirect.com/topics/chemistry/polyester-fiber
Hann S, Sherrington C, Jamieson O, Hickman M, Bapasola A (2018) Investigating options for reducing releases in the aquatic environment of microplastics emitted by (but not intentionally added in) products, Final Report, Report for DG Environment of the European Commission, London
Harrison J (2018) Microfibers n the freshwater environment, https://neiwpcc.org/information-center/neiwpcc-reprint-series/microfibers-frewshwater-plastic-fibers/
Jemec A, Horvat P, Kunej U, Bele M, Krzan A (2016) UPTAKE and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environ Pollut 219:201–209
José S, Jordao L (xxxx) Exploring the interaction between microplastics, polycyclic aromatic hydrocarbons and biofilms in Freshwater, https://www.tandfonline.com/doi/abs/https://doi.org/10.1080/10406638.2020.1830809?journalCode=gpol20
Kärkkäinen N, Sillanpää M (2021) Quantification of different microplastic fibres discharged from textiles in machine wash and tumble drying. Environ Sci Pollut Res 28(13):16253–16263. https://doi.org/10.1007/s11356-017-0528-7
Kershaw PJ (2015) Sources, fate and effects of microplastics in the marine environment: a global assessment. International Maritime Organization, London
Lee H, Shim WJ, Kwon JH (2014) Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci Total Environ 470–471:1545–1552. https://doi.org/10.1016/j.scitotenv.2013.08.023
Li Z, Xialin Hu, Qin L, Yin D (2020a) Evaluating the effect of different modified microplastics on the availability of polycyclic aromatic hydrocarbons. Water Res 170:115290. https://doi.org/10.1016/j.watres.2019.115290
Li M, Yu H, Wang Y, Li J, Ma G, Wei X (2020b) QSPR models for predicting the adsorption capacity for microplastics of polyethylene, polypropylene and polystyrene. Sci Rep 10:14597. https://doi.org/10.1038/s41598-020-71390-3
Palacios-Mateo C, van der Meer Y, Seide G (2021) Analysis of the polyester clothing value chain to identify key intervention points for sustainability. Environ Sci Eur 33:2. https://doi.org/10.1186/s12302-020-00447-x
Pittura L, Avio CG, Giuliani ME, d’Errico G, Keiter SH, Cormier B, Gorbi S, Regoli F (2018) Microplastics as vehicles of environmental PAHs to Marine organisms: combined chemical and physical hazards to the mediterranean mussels mytilus galloprovincialis. Front Mar Sci. https://doi.org/10.3389/fmars.2018.00103
Qi R, Jones DL, Li Z, Liu Q, Jones DL, Li Z, Liu Q, Yan C (2020) Behavior of microplastics and plastic filmresidues in the soil environment: a critical review. Sci Total Environ 703:134722
Smol M, Włodarczyk-Makuła M (2017) The effectiveness in the removal of pahs from aqueous solutions in physical and chemical processes: a review. Polycyclic Aromat Compd 37(4):292–313. https://doi.org/10.1080/10406638.2015.1105828
Sørensen L, Rogers E, Altin D, Salaberria I, Booth A (2020) Sorption of PAHs to microplastic and their bioavailability and toxicity to marine copepods under co-exposure conditions. Environ Pollut 258:113844
Velzeboer I, Kwadijk CJ, Koelmans AA (2014) Strong sorption of PCBs to nanoplastics, microplastics, carbonnanotubes, and fullerenes. Environ Sci Technol 48:4869–4876
Wang F, Zhang M, Sha W, Wang Y, Hao H, Dou Y, Li Y (1827) Sorption behavior and mechanism of organic contaminanst to nano and microplastics. Molecules 2020:25
Włodarczyk-Makuła M, Wiśniowska E (2018) Decomposition of carcinogenic hydrocarbons in an integrated oxidation–sorption system. Annu Set Environ Prot 20:713–724
Ziccardi LM, Edgington A, Hentz K, Kulacki KJ, Driscoll SK (2016) Microplastics as vectors for bioaccumulation of hydrophobic organic chemicals in the marine environment; a state-of-the science review. Environ Toxicol Chem 35(7):1667–1676. https://doi.org/10.1002/etc.3461 (Epub 2016)
Funding
The study was funded by the Statutory Funds of Czestochowa University of Technology BS-PB-400–301/21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no conflicts of interest to declare that are relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wiśniowska, E., Włodarczyk-Makuła, M. Evaluation of the adsorption efficiency of carcinogenic PAHs on microplastic (polyester) fibers—preliminary results. Appl Water Sci 12, 119 (2022). https://doi.org/10.1007/s13201-022-01654-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01654-y