Abstract
Uranium (U) is formed by the interaction of groundwater in aquifer and the surrounding rocks. The area chosen for this study is a hard rock terrain of various lithology in South India, which releases U in groundwater and affect the groundwater quality. Hence, this study was conducted to understand the geochemistry and temporal variations of U in groundwater with respect to saturation state of minerals for different seasons namely Pre-monsoon (PRM), Northeast monsoon (NEM), Southwest monsoon (SWM) and Post-monsoon (POM). A total of 216 groundwater samples were collected, representing various lithology (Fissile hornblende biotite gneiss, Charnockite, Quartzite, Granite and Flood Plain Alluvium) in the study area. The collected samples were analyzed for physical parameters such as Electrical conductivity, Total dissolved solids, pH and major ions. U was measured using Laser Fluorimeter. The study infers that weathering in SWM, ion exchange in PRM and POM and anthropogenic process in NEM were the three major processes that could dominate the hydrogeochemistry of U. The higher concentration of U has been highlighted, and the water–rock interaction has been studied to know the sources of origin and it has been plotted through bivariate and ternary diagrams. The saturation index study was made and calculated for uranium, carbonate, sulfate, silicate and phosphate minerals using PHREEQC Programme. Uraninite and Coffinite are the dominant saturated states of U minerals which tend to attain near saturation to saturation state irrespective of seasons. With the increase in U concentration irrespective of seasons, it was noticed that the saturation state of minerals is also increasing. Overall, the weathering process and the lithological impact are the sources for higher concentration of U and their minerals occurring in the study area. The outcome of the study will help the policy makers for sustainable management to safeguard the groundwater resource in this region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hard rock in the aquifers with fractures, joints and porosity affects the quality, storage and groundwater flow. U being hazardous to the environment, found naturally in surface and groundwater systems. The concentration of 2.7 ppm of U is reported as an average value in earth crust (Siegel and Bryan 2004). The solubility of radionuclides governs the distribution and dispersion in water (Jordana and Batista 2004). Studies have reported U level in groundwater used for drinking purpose (De Camargo and Mazzilli 1996; Chau and Michalec, 2009; Killiari and Pashalidis 2010; Thivya et al. 2015a, 2015b; Brindha et al. 2011a, b; Cho and Choo 2019). The studies have also tried to trace the geochemical processes behind the release of U in groundwater (Mahalakshmi et al. 2017, Paramaguru et al. 2016; Chandramohan et al. 2018; Adithya et al. 2019, 2020; Selvi et al. 2016). U occurs in certain types of rocks in three different forms as background, resistant and interstitial modes (Guthrie and Kleeman, 1986; Gueniot et al. 1988). It exhibits IV+ and +VI+ oxidation states in rock and aqueous forms. Reduction of U6+ to U4+ is the desired deletion mechanism, with the resultant of U4+ species in a lesser amount of soluble and thus not as much transportable in groundwater (Fiedor et al. 1998), presumptuous that the U4+ species is not colloidal. Thus, the solubility of U in groundwater acting a significant part when making an allowance for actual approaches for its remediation. Silicic acid commonly occurs in mineral soils, at concentrations of about 10−3 mol/L (Wilding et al. 1977). The following reactions describe the dissolution of amorphous silica (1), the dissociation of silicic acid (2), and the complex formation with uranyl (3).
U ion generally occurs in tetravalent and hexavalent forms. The mobility of U in groundwater is mainly dependent on the oxidation state and also higher concentrations are found in toxic waters (Osmond and Cowart 1976; Rajesh Kumar et al. 2015). Since chemical composition varies, the geochemistry of groundwater is more vital as the dependency of groundwater increases due to increase in population, agricultural practices, and rapid development of industries (Yidana et al. 2008; Prasanna et al. 2011; Chidambaram et al. 2011, 2020). U in groundwater of the aquifer changes its characteristics while moving along its path from recharge to discharge regions, due to hydrogeochemical processes (Thivya et al. 2015a).
The hexavalent U ion is more vulnerable to the contamination of the groundwater by diffusion and advection processes (Bucur et al. 2006). Metallic U is chemically reactive and can be leached into the groundwater in acidic or alkaline environment. While in an alkaline environment, it strongly gets adsorbed into the groundwater (Hsi and Langmuir 1985). U in groundwater is mainly governed by ORP conditions and also by pH (Langmuir 1997). The available U in particulate and dissolved sources in the form of secondary minerals are Uraninite, Pitchblende, and Cornlite in the complex forms as silicates, phosphates, carbonates (Shabansefidan and Anji Reddy 2012). Radioactive nuclides in groundwater have unique physical and chemical characteristics like solubility (Jordana and Batista, 2004), mobility and half-life; varies from the parental isotope in its amounts and its distribution changes (Zapecza and Szabo 1986; Langmuir 1997; Senior 1998; Adithya et al. 2019; Reynolds et al. 2003). The concentration of free radionuclide ions in water can change because of hydrolysis and may lead to precipitation or adsorption; or complexation due to interaction with ligands CO32−, Cl−, SO42− and NO32−. The process of weathering of the mineral in the host, rock may change the physical characteristics in terms of grain size, growth habit, and mineral association. The concentration of U in the environment is governed by the geochemical mobility subsequent to the interaction with the rock (Tieh et al. 1980; Ali et al. 2011). During extensive leaching of the host rock, the interstitial U will be further weathered by ion-exchange and adsorption processes. A study on the geochemical evolution of groundwater is important to sustain water resources and also to understand groundwater quality due to the release of radio nuclides, Thivya et al. (2015b) studied the seasonal variations of U and their relationship with isotopes and speciation, but the study has been limited to a few samples.
The saturation states of minerals in groundwater reflect the thermodynamic nature of the composition. Thus it helps to unravel the reaction history and the current geochemical character with a focus on future reaction pathways. It is practically impossible to manually calculate the species distribution by applying the principle of thermodynamics. Various phases in solid, liquid, and gaseous forms are generally considered to understand the interactions in a geochemical system. To overcome the impracticality, various specialized computer programs were developed considering these principles, each has its own advantages and limitations. The most commonly used models include PHREEQC (Parkhurst and Appelo 1999), MINEQL (Westall, and Zachary 1986), WATEQ4F (Trusdell and Jones 1973) and JCHESS (Van der Lee 1998). In these thermodynamic equilibrium studies, the evolution of compositional stability is brought out by understanding the saturation states. This is mainly achieved by the molar concentration of the reactant and products through the solubility constant (Ksp). The solubility constant of a reaction is then compared with ionic activity product (IAP), determined from the mole concentration of ions analyzed from the solution.
It has been reported that higher amounts of U are present in the groundwater of Madurai district in Tamilnadu, South India, i.e., vary from 12 to 28 ppm (Pandey 1995), 113 ppb during post-monsoon (Thivya et al. 2015a, 2015b) and 46.70 ppb during north east monsoon (Thivya et al. 2014). Even the previous studies have reported the U concentration in groundwater, but they are not concentrating the influence of saturation states of different minerals to the release of U. Hence, the present study is so significant because the prominent levels of U detected in the groundwater affect human health. In addition to that, this study also discusses the geochemical behavior of U in groundwater and its temporal variations. We executed the saturation index of uranium minerals to evaluate the relationships among U and saturation state of carbonate, silicate, sulfate and phosphate minerals in groundwater of Madurai region, South India.
Study area
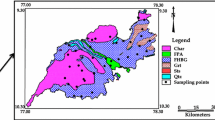
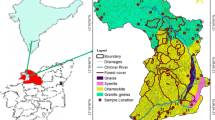
Madurai district covers a total area of 3741 km2 between latitude 9°30ʹ–10°30ʹN and longitude 77°0ʹ–78°30ʹE. It is located in the southwestern part of Tamilnadu bounded by Sivagangai in the East, Theni on the west, Tiruchirapalli and Dindigul districts in the north, and Virudhunagar district in the south (Fig. 1). The lithological study contains fissile Hornblende Biotite Gneiss, Quartzite Granitic intrusions, Floodplain Alluvium and Charnockite (GSI 1995; Thivya et al. 2013a, 2015b) among which the fissile hornblende gneiss and the Charnockite represents the major lithology of the study area. The district is characterized by Red soil, Black clayey soil and Alluvial soil. Red soil is found in all the blocks of the district while Black clayey soil is found in Tirumangalam, Usilampatti and Peraiyur blocks of the district and alluvial soil is found along with the courses of the river. Study region located in the southern granulite terrains. Higher mafic forms of massive Charnockites are detected in this region with the mineral assemblage of clinopyroxene, orthopyroxene, hornblende and plagioclase. The khondalite also contains potash feldspars, cordierite, garnet, sillimanite, quartz, spinel, biotite and plagioclase, with graphite, apatite and zircon as major accessories (Baiju and Nambiar 2006; Thivya et al. 2015a, b).
Vaigai is the major river flowing along the NW–SE separating the district into two and drains an area of 1615.39 km2. Long-term analysis of rainfall for a period of 100 years from 1904 reveals that NE monsoon rainfall is the major source of precipitation contributing 47%, the next predominant rainfall season is from June to September (SW Monsoon) contributing about 32% of rainfall followed by 17% during summer. The district is predominantly with agricultural practices and it is initiated during the southwest monsoon. Paddy and sugar cane cultivation are the main crops in this district. The cultivable area of this district is 205,674 ha, of which 48,631 ha (nearly 23.65% of the area) have irrigation facilities from sources like canals, tanks and wells. The groundwater is observed to be shallow along the river and in the flood plain. The water level fluctuates among the seasons and the deepest is observed during summer. The flow of groundwater is observed to be from SW to NE and Southern to NE direction towards the river (Thivya et al. 2015a).
Methodology
Groundwater samples were collected from the hand pumps in different seasons, viz. Post-monsoon (POM), Southeast monsoon (SWM), Premonsoon (PRM), and Northeast monsoon (NEM) (Fig. 1). A total of 216 samples were collected and each sample quantizes to 1L covering the temporal variation and lithology. Few parameters like total dissolved solids (TDS), electrical conductivity (EC), pH were analyzed in the field by a portable Themo Orion 5-star meter. The samples were closed tightly and transported to the lab and refrigerated at 4 °C. To have good support for the study and its comparison with major ions, the analysis of Ca, Mg, HCO3 and Cl were estimated by titrimetry. Na and K were determined by ELICO CL 378 flame photometer for the samples with values ranging from 1 to 100 mg/L. the samples with values > 100 mg/L were diluted and analyzed, with a precision of ± 1. Silica (Silica molybdate method), phosphate (Orthophosphate method), and sulfate (SulfVer4 method) were analyzed by spectrophotometric technique (HACH DR5000), with an accuracy of ± 2.5 nm, (APHA 1995; Ramesh and Anbu 1996).
U is measured as fluorescence in laser fluorimeter at a wavelength of 337 nm, frequency 10 Hz using an exciting source of nitrogen laser with a measurement range of 0.2–20 ppb (Fig. 2) with a pulse energy of 20 µJ with a duration of 7 ns and small sample size (3–5 mL). 1.789 g of (CH3COO)2UO2·2H2O was used to prepare the stock solution by dissolving the salt in 1000 mL of water with 1 mL of 70% pure HNO3. The stock solution was used to calibrate with a fluorescent agent (5% phosphoric acid) to detect the uranium level in the water. The blank solution was also measured for Uranium with the same quantity of the fluorescent agent.
Piper plot is a graphical way of visualizing the water types. Aquachem software was adopted to plot the piper diagram. The saturation index study was attempted using the geochemical program PHREEQC (Parkhurst and Appelo 1999). The ionic activity product (IAP) is obtained from the product of the activities of chemical constituents in solution at a definite temperature. Similarly, the solubility of mineral calculated for a definite reaction is modified with respect to temperature and considered as Ksp (Merkel and Planer-Friedrich 2005). Later the log ratio of IAP to Ksp provides the state of saturation (SI), for specific chemical composition (mineral). Based on this value, the reaction is considered to be at equilibrium (SI = 0), or under saturated (SI < 0) or over -saturated (SI > 0). Thus the values determine if the solution will react with the solid mineral phase of the same composition along with its pathway or in situ reactions. PHREEQC is a software programme used to measure the saturation index based on an aqueous ion association model for groundwater chemical composition (Parkhurst and Appelo1999) and believed to be indicative of groundwater chemical evolution. The saturation index of uranium, carbonate, sulfate, silicate and phosphate minerals was calculated in the present study.
Results and discussion
pH values measured for the samples ranging from 5.2 to 8.2 which is acidic to alkaline. One of the samples in POM and NEM was observed with the lowest and highest pH. Higher values of EC were observed along the Vaigai river course irrespective of the season, and it was attributed to the influence of domestic sewage outlet into the river (Thivya et al. 2013a). The values of EC vary between 107.66 and 6850 µs/cm. The order of dominance of ions is given in Table 1.
Uranium levels in groundwater
The concentration of U increases with monsoonal changes and the highest was observed in POM (Fig. 3). The maximum values with respect to different seasons follow the order of dominance as SWM > PRM > POM > NEM. The concentration of U was above 30 µg/L irrespective of all seasons and the highest was observed in Karungalakudi granitic terrains. Seasonally, one sample in SWM, 2 in POM and 3 in NEM were above the permissible limits (WHO 2012).
The bones and kidney are the chief locations for the accumulation of U, whereas skeleton is the foremost spot for the long-term storage of U (Morrow et al. 1982; Zamora et al. 1998; Wrenn and Singh 1982). When U enters the body, 80% are excreted through urine and feces, 10% are deposited in the kidneys and skeleton (NRC 1983). The proximal tubules in the kidney are the main part damaged by U (Mao et al. 1995; Zamora et al. 1998). The study by Kurttio et al (2002) suggests that chronic uranium exposure in the human is mainly associated with increases in blood pressure. Even lower concentration of U in drinking water also causes the nephrotoxic effects. For the sustainable management of polluted groundwater, various techniques are used such as membrane filtration methods, anion exchange, and the use of adsorbents, such as iron oxides, or titanium dioxide, as well as the application of coagulation processes with the addition of Fe/Al salts, or by lime softening methods to remove the U from groundwater.
Basically, it was observed that the U concentration varies in groundwater while the recharging and discharge process. The recharge process that occurs via rainfall would increase the groundwater level. This helps to the dissolution of U through the interaction of aquifer rock weathered in the unsaturated zone and increases its concentration in groundwater; observed especially in SWM. However, a converse result is observed in NEM is that, as the recharge continues, the concentration of U in groundwater begins to reduce due to anthropogenic activities.
Hydrogeochemical processes
Hydrochemical facies plays a vital role in understanding the complex hydrochemical processes in the subsurface of the aquifers and can be used for the chemical assessment of groundwater and surface water qualitatively, the origin of water, and pattern of flow. Few attempts were made by Hill (1940) and Piper (1944) in the year for the modification of the hydrogeochemical theory and came up as Piper plot. Further, the piper plot was improved by Durov and Akad Nauk (1948), Johnson et al. (1970) as a diamond field, and finally by Chadha (1999). Thereafter the piper plot came into the limelight in the form of software packages.
Piper plot was drawn for the present study using the major cations and anions (Ca2+, Mg2+, Na+, K+, HCO3−, SO42− and Cl−) of the groundwater samples which are measured in equivalent per million. The ions were plotted in the form of triangular and projecting out at the center as a diamond field. The characteristics of the groundwater were known from the piper plot.
In PRM, the ion exchange and anthropogenic activities dominated as most of the samples were clustered in the Mixed Na–Ca–HCO3–Cl type (Fig. 4) and Na–HCO3–Cl type and few representations are of Ca–HCO3–Cl type (Srinivasamoorthy et al. 2011). The geochemical type of SWM groundwater reflects recharge conditions (Prasanna et al. 2010) as Ca–HCO3 type was predominant and some of the samples of SWM are scattered in the mixed water type. The anthropogenic impact is more predominant in NEM because most of the samples are Na–Cl type and some samples fall in Ca–Mg–Cl type. Leachate may occur in POM. This is observed from the piper plot by the formation of the mixed type of cations and anions Ca–Na–HCO3 are distinctive of granite aquifer with feldspars (Cho and Choo 2019) and also Ca–Cl (Chidambaram et al. 2012).
Three mechanisms were identified based on the seasonal changes.

In PRM, association of Na–K–HCO3–Cl observed was mainly due to weathering or ion exchange processes represents like feldspar. In the first mechanism, the release of Ca or absorption of Na in groundwater takes place while changing from PRM to SWM. Later in SWM, the concentration of HCO3 ions increased due to the dissolution and weathering of minerals. And also the recharge process dominates. In NEM, Anthropogenic processes overcome the weathering or ion exchange process in the second mechanism which is due to dissolution or leaching of ions in groundwater, and therefore, Na–Cl type is predominant. The third mechanism identified during POM, the ion exchange, and weathering dominate. A water type of Ca–Na–HCO3–Cl type reflects mixed water conditions. Therefore, it is inferred that the increase in Na levels leads to a decrease in Ca ions due to the interactions between aquifer matrix and groundwater.
Association and behaviour of U ion
To understand the migration of U ions in groundwater, a piper diagram was plotted between the hydrogeochemical parameters and U concentration (Fig. 5). The migration of ion type was accordingly to seasons viz. Na–Ca–Cl–HCO3 (PRM) to Na–Mg–Cl–HCO3 (SWM) to Na–Cl (NEM) to Mixed Na–Mg–Cl–HCO3 (POM). If the Na/Cl ratio is increased, then the simultaneous decrease of (Ca + Mg)/HCO3 ratio takes place. The ion exchange mechanism clearly shows that the removal of Ca and Mg in groundwater is exchanged for the Na and K released from the adsorbed clay surfaces. The source of Na would be due to the weathering of Plagioclase feldspar. During SWM Na–Mg–Cl–HCO3 exists due to the weathering process in which Ca is removed from the system and Mg is added during this monsoon due to dissolution of Mg-rich minerals from Charnockite rock along the flow path. SWM shows higher U due to rapid recharge processes. This increased shallow water level and increased the toxic nature of groundwater resulted by enhancement of U concentration through the recharge process (Thivya 2014). In NEM, dissolution; weathering; and anthropogenic activities result in Na–Cl type, and a low concentration of U was noted. In this season Mg and HCO3 were removed due to anthropogenic processes. In POM, Mg and HCO3 were contributed as mixing processes evolved after the monsoon the uranium levels were higher in the regions represented by Charnockite rock. The factors that influence the enrichment of major and trace elements are mineralogical composition, weathering, particle size, pH, leaching, and human activities. The elements in groundwater are mainly governed by the process of mineral weathering and their spatial distribution, which is further controlled by their geochemical mobility.
Effect of pH and ORP with U
The concentration of U varies with respect to pH (Fig. 6). U above 30 µg/L was noticed when pH is neutral (Jurgens et al. 2009a; Thivya et al. 2015a, 2015b). Also, a trend that low concentration of U was identified when the pH increases. The positive value of ORP exhibits oxidation processes and negative value reflects reduction processes. The concentration of U increases or decreases depending on the redox reaction it undergoes. Deposition of U takes place when it tends to undergo reduction and lowers Eh (Brindha et al. 2011a, b). The ORP values vary between 71 and 245 mV irrespective of seasons (Fig. 7) indicates the oxidation processes which enhanced the U in groundwater (Cowart and Osmond 1977; Toulhoat and Beaucaire 1991). There is no linear trend observed with ORP and uranium.
Effect of bicarbonate with U
Weathering process increases the bicarbonate concentration in groundwater (Srinivasamoorthy et al. 2008). High concentrations of U are associated with shallow, groundwater that has high concentrations of bicarbonate. U levels in groundwater are also mainly governed by the availability of HCO3 ions in solution. There are two cases observed in the higher concentration of U: 1. Lower HCO3 contains higher uranium concentration; 2. Higher HCO3 contains higher uranium concentration. The seasonal variations of HCO3 with respect to U are observed in the plot (Fig. 8). In PRM, HCO3 concentration decreases with a decrease in U content in most of the samples, whereas in all other seasons HCO3 concentration increases with U content in groundwater. In general, higher U content shows that a clear increasing trend with HCO3 irrespective of seasons.
Ion exchange process
The interaction of groundwater and its hard rock of the aquifer undergo ion exchange process seasonally which increased the Na. Only Ca is the dominant ion in SWM whereas Na is the dominant ion in all other seasons (Fig. 9). Ca is replaced by the Na ions according to the equation below.
The higher concentration of U samples falls in the Na dominant region that indicates the liberation of this ion increases the U concentration in groundwater. Only one sample falls in Ca dominant in SWM as Ca is the dominant ion in this season. A study was reported that U concentration increases in an alkali matrix that helps in leaching and migration in groundwater (Dressen et al. 1982). Na concentration in groundwater above equiline indicates that it may be evolved during the process of cation exchange and alkali mineral weathering (Thilagavathi et al. 2014; Nur and Ayuni 2011).
Weathering process
The process of percolation or infiltration, dissolves the metals present in the hard rock of the aquifer which increases the Ca and Mg in groundwater. The studies made in the form of scatter diagram by Datta and Tyagi (1996) for (Ca + Mg) versus (HCO3 + SO4) indicate carbonate weathering if the samples fall above the equiline. Most of the sample points fall below the equiline. It is to be noted that few points fall along and above the equiline with few along the equiline and above (Fig. 10). Since most of the samples fall below the equiline, it is inferred that the predominant process is not carbonate weathering (Dehnavi et al. 2011). The mechanism involved during the weathering process could be as follows
The reaction explains the release of Ca from the Calcite due to the reaction with H2CO3. The pCO2 enriched water in the acidic condition serves as an extensive source of solution for carbonate weathering. Thus it helps in the dissolution and reaction of carbonate minerals along its flow direction.
Also bicarbonate can react from the calcite of aquifer rock and increases Ca and Mg in groundwater.
Silicate weathering takes place from Albite and bicarbonate
So, the study indicates that samples with higher uranium concentration in PRM and SWM samples fall in silicate the weathering region, whereas in NEM two samples fall in carbonate weathering and one sample in silicate weathering. In POM also one sample falls in carbonate and one sample in silicate weathering. Therefore, the bicarbonate ions increase with monsoon and thus enhances uranium (Dehnavi et al. 2011; Jurgens et al. 2009b).
To determine the exact sources of uranium concentration whether it has been originated from weathering or by anthropogenic processes, a different study like the bivariate plot has been attempted between Ca + Mg + Na + K/Cl + SO4 with Cl + SO4 in milliequivalents (Kim et al. 2005) as Ca, Mg, Na, K are the major ions that control the geochemistry of groundwater. The concentrations of Cl and SO4 acted as an indicator for the anthropogenic contamination of groundwater (Lowrance et al. 1997; Thunqvist 2004). The same was noticed along the Vaigai river course (Thivya et al. 2013a, b, 2014, 2015a, b) and higher sulfate concentration was noticed in the study which could be due to leaching of fertilizer (Thivya et al. 2013a). The study also revealed that most of the samples move along the vertical line which indicates the mineral weathering processes irrespective of seasons (Fig. 11). Few of the samples move along the Cl + SO4 indicating the anthropogenic process. The samples with higher U concentration plotted separately also indicate the possibility of mineral weathering processes.
To determine the characteristics of U in groundwater, a ternary plot between the cations (Ca–Mg–Na + K) were plotted (Fig. 12). It was observed that most of the samples fall and move towards the Na + K apex where Na is the dominant ion in all seasons except SWM. The ternary diagram (HCO3 + CO3) to (Cl + SO4 + NO3) to (H4SiO4) has been constructed as to understand the relationship of bicarbonate, silicate and chloride, sulfate and nitrate which origin from the anthropogenic processes. Zone I and II are classified by the weathering processes and zone III as anthropogenic processes dominant region. The higher concentration of U samples falls in weathering regions, whereas one sample of PRM and NEM is due to anthropogenic process, because in NEM Na–Cl type is dominant whereas in PRM Na–Ca–Cl–HCO3 type dominates.
Saturation index (SI) of uranium minerals
The redox potential governs the mobility of uranium, oxidation promotes dissolution and reduction leads to precipitation of secondary minerals. The chief secondary minerals thus dissolved are mainly related to oxides or carbonates of uranium (Burns and Finch 1999). SI of Uranophone, Rutherfordite, Schoephite, Uraninite, and coffinite are the chief U minerals that were considered for the study (Fig. 13). Irrespective of the season the near saturation to saturation states of uraninite and coffinite were observed. The thermodynamic state of undersaturation was observed for other minerals like Uranophone, Rutherfordite, Schoephite, UO2 and UO3. It is interesting to note that the increase in U increases the saturation states of U minerals irrespective of the season.
U in groundwater is mainly dependent upon the dissolution of primary and secondary minerals (Langmuir 1997). Groundwater at reducing condition at shallow depths reflects the saturation states, but at deeper levels show undersaturation. The increasing trend is observed with schoepite minerals which move from undersaturation to saturation state. Uraninite generally weathers to Schophite at an early stage, but it does not control the phase solubility in long term (Finch and Ewing 1992). When the schoepite is exposed to higher levels of phosphate in solution, it undergoes changes to several intermediate uranyl phosphate compounds and its transformed Autunite (Sowder 1998). The above-said processes are active along the ground water flow direction, which leads to a significant change in geochemistry due to rock water interaction. According to Jordana and Batista (2004), at high pH anions and oxy-anions is more mobile whereas most cations are less mobile, but at low pH this tendency reverses. It is difficult to precisely determine the analytical value of Uranium in a few critical conditions since there exists a significant variation in the solubility of uranium minerals. Hence, due to this fact the stability of uranitie and few chief uranium minerals considered is governed by the presence of impurities in the solution (Meinrath et al. 1999).
Uraninite has the chemical formula UO2, but invariably in the environment some of the U is oxidized to U (VI), and therefore the formula can also be expressed as UO2 + x, where x < 0.3. Coffinite has been found to be a common alteration product of Uraninite in Si-rich reducing environments (Janeezek 1991; Janeezek and Ewing 1992). In oxidizing environments, precipitation and dissolution of secondary minerals alter Uraninite into several minerals. Solubility is greatly enhanced in oxidative environments containing carbonates for complexation.
SI of carbonate minerals compared to U
The saturation state of carbonate minerals like SIA (Aragonite), SIM (Magnesite), SID (Dolomite) and SIC (Calcite) was compared with Uranium (Fig. 14). Sprinkle (1989) proved that due to the errors in measurement of pH and alkalinity titration along with CO2 variations in pCO2 values due to outgassing of CO2, the SI values ranging between − 0.2 and + 0.2 are considered to be in equilibrium for calcite and from − 0.4 to + 0.4 for dolomite. Uranium for UO2 and Calcite are released into the solution then from other minerals. Similarly, CO3 species forms a typical complexation forms with U in solution depending on the pH, ORP, and availability of HCO3 ions in water.
The saturation index of zero implies equilibrium, but a saturation index of − 0.2 to + 0.2 can be considered to be representative of Calcite saturation and − 0.4 to + 0.4 for Dolomite saturation because of the probability of CO2 outgassing and pH and alkalinity errors. When U is kept in minerals like uraninite (UO2) or calcite (CaCO3) in suitable circumstances it can transfer from the rock to groundwater. Another ubiquitous aspect of groundwater that forms strong aqueous complexes with UO22+ is carbonate. In PRM and POM the higher concentration of U corresponds to the state of over saturation state of calcite and aragonite in an equilibrium state, Dolomite in near saturation state and magnesite is observed in undersaturation state. Most of the Calcite minerals are oversaturated and increasing trend of U with SI of Carbonate minerals are noted. The order of dominance of saturation index in PRM SIC > SIAr > SIDo > SIMg. The order of dominance of SI in SWM is SIMg > IiC > SIDo > SIAr. The order of dominance of SI in NEM is SIC > SIAr. State of saturation decreases during NEM and SWM, it is due to an increase of groundwater recharge and dissolution of rock matrix of the groundwater chemistry (Karmegam 2012). The higher SI of calcite and aragonite noted between 0.01 and 0.001 m of U concentration. Still, the saturation index of these minerals decreases with increased U concentration. This may be due to the fact that the emerging HCO3 resulting from the weathering reaction might be attracted towards ‘U’ then towards Ca or Mg ion which thereby reduces the SI of carbonate minerals (Calcite, Dolomite, Magnesite, Aragonite). The increase of groundwater recharge and the dilution of groundwater chemistry with respect to the minerals of calcite and dolomite after the subsequent monsoon periods are due to the non-availability of Ca (Karmegam 2012). The saturation index decreases in NEM and SWM with respect to uranium, it is due to the increase of groundwater recharge and dissolution of the rock matrix of the groundwater chemistry (Thivya 2014). There is a further decrease in saturation state, due to the removal of released HCO3 by complexation with uranium than towards Ca or Mg ion which is thereby reflected in the reduction of SI of carbonate minerals (Calcite, Dolomite, Magnesite, Aragonite). The development of uranyl-carbonate complexes renders U to be highly mobile, which is mainly governed by the concentration of carbonates in solution.
SI of sulfate minerals compared to U
The sulfate minerals like Gypsum and Anhydrite are under saturation state in all seasons. Equilibrium conditions were observed with respect to SI of gypsum in the regions with fresh groundwaters (Fig. 15) (Plummer et al. 1990). PRM shows higher values of SO4 in groundwater and the saturation states of these two different minerals represent the following trend SIGy > SIAn and the SI of SO4 minerals reflect under saturation irrespective of the season. It is evident that SI of sulfate minerals increases with an increase in U concentration in all seasons except that of PRM which is mainly due to the anthropogenic influence.
SI of silicate minerals compared to U
Silica reactions are likely to be more complex and varied, whereas stoichiometric reactions seem basic. The equilibrium reaction can be written as,
The silica minerals like Silica Gel, Chalcedony, and Cristobalite represent the amorphous, cryptocrystalline and crystalline forms respectively (Fig. 16) and they are referred to as SIcr, SIch SIsg. The saturation of these silicate minerals were calculated. In PRM the SI of Chalcedony and Cristobalite is noted as oversaturation state, whereas silica gel is observed in near saturation state. The orders of the dominance of SI minerals are SIcr > SIch > SIsg for all seasons. The saturation index of all minerals irrespective of the season does not show any definite trend with U concentration.
SI of phosphate minerals compared to U
Higher concentration of uranium is also observed due to the weathering of apatite (Murray et al. 1983; Arey et al. 1999), atunite (U-phosphate) and form fertilizers (Cothern and Lappenbusch 1983; Jerden et al. 2003). The reaction of U(VI) ion with the PO4 ions in solution can lead to the formation of atunite as the PO4 is released into the solution by weathering of apatite (Christopher et al. 2003). Sumner (2000) advocates the formation of hydroxylapatite in different stages, initially from amorphous to crystalline, followed by transformation of di to octa calcium phosphate and then finally to hydroxylapatite. Various studies have suggested that phosphate minerals are often a potential contaminate of U groundwaters irrespective of the season. The higher concentrations of U with hydroxyapatites are under saturation state whereas in SWM it is observed in near saturation state (Fig. 17). The higher concentration of PO4 is observed in NEM and subsequently, U is noted to be lower in this season. Studies have also proved that the groundwater is polluted by the U due to the dissolution of phosphatic minerals.
Conclusion
The outcome of the present study shows that the average concentration of U increases with seasonal changes especially during monsoons and the highest concentration was observed in POM. The maximum values observed for U in all seasons are as follows; SWM > PRM > POM > NEM. The piper plot results inferred that PRM samples are inclined to ion exchange/chemical weathering processes whereas the recharge and weathering processes are dominated in SWM. Influence of anthropogenic and ion exchange processes in NEM leads to the Na–Cl type due to the dissolution process and finally both monsoon and non-monsoonal characteristics were reflected in POM. The samples containing U concentration greater than 30 µg/L in the piper plot observed that, the process is controlled by the geochemical nature of the groundwater. On comparison of HCO3 and U, it was identified that higher U concentration irrespective of HCO3 varied seasonally. The presence of alkali matrix increases the U concentration which helps in leaching and migration in groundwater. The higher concentration of U was found due to weathering processes and aquifer matrix mixing by anthropogenic sources and acts as a dominant ion. The reduction of SI of calcite, dolomite, magnesite and aragonite is due to the removal of released HCO3 by complexation with U than towards Ca or Mg ion. SI of sulfate minerals increases with the increase in U concentration due to the anthropogenic influence. The saturation index of Uraninite and Coffinite is dominant, and it also tends to attain the near saturation to saturation state. Hence, it was observed that uranium minerals vary with the concentration of total U in groundwater. Further, weathering is a predominant process in the study area which regulates the saturation state of minerals in groundwater. Since U concentration in few samples showing above the permissible limit irrespective of seasons, there is a need to safeguard the water resource through feasible sustainable management plans.
Data availability
The manuscript and data are the authors' original work, and the manuscript has not received prior publication and is not under consideration for publication elsewhere. The manuscript now submitted is not a copied or plagiarized version of some other published work.
References
Adithya VS, Chidambaram S, Tirumalesh K, Hemant M, Prasanna MV (2019) Occurrence of Uranium in groundwater along the lithological contacts in central Tamilnadu, India: an isotope hydrogeochemical perspective. Expo Health 11:277–290. https://doi.org/10.1007/s12403-017-0269-3
Adithya VS, Keesari TK, Thivya C, Thilagavathi R, Venkatramanan S, Chidambaram S, Prasanna MV (2020) Seasonal changes in groundwater quality deterioration and chemometric analysis of pollution source identification in South India. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-08258-6
Ali M, Shariff AA, Qamar NA, Laghari A (2011) An appraisal of uranium source potential of granites, associated felsic rocks, kaolin and calcretes of Nagar Parkar area, Tharparkar Pakistan. J Himal Earth Sci 45(2)
APHA (1995) Standard methods for the examination of water and waste water, 19th edn. APHA, Washington DC
Arey JS, Seaman JC, Bertsch PM (1999) Immobilization of uranium in contaminated sediments by hydroxyapatite addition. Environ Sci Technol 33:337–342
Baiju KR, Nambiar C (2006). Metamorphism and fluid inclusion studies of charnockites and associated rocks from the Madurai granulite block, southern India with special reference to graphite mineralization
Brindha K, Rajesh R, Murugan R, Elango L (2011a) Fluoride contamination in groundwater in parts of Nalgonda district Andhra Pradesh India. Environ Monit Assess 172:481–492
Brindha K, Elango L, Nair NR (2011b) Spatial and temporal variation of uranium in a shallow weathered rock aquifer in southern India. J Earth Syst Sci 120(5):911–920
Bucur C, Olteanu M, Pavelescu M (2006) Radionuclides diffusion in geological media. Romania J Phys 51(3–4):469–478
Burns P, Finch R (1999) Uranium mineralogy, geochemistry and the environment. Reviews in mineralogy. Mineral Soc Am 38:1–679
Chadha DK (1999) A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol J 7:431–439
Chandramohan J, Ganesh D, Harikrishnan N, Ravisankar R, Chandrasekaran A, Neelakantan MA, Raja V (2018) Study of Uranium concentration in drinking water in Tiruvannamalai district, Tamil Nadu, India and assessment of health risk. J Rad Nucl Appl 3(2):103–110
Chau ND, Michalec B (2009) Natural radioactivity in bottled natural spring, mineral and therapeutic waters in Poland. J Radioanal Nucl Chem 278:121–129
Chidambaram S, Karmegam U, Prasanna MV, Sasidhar M, Vasanthavigar M (2011) A study on hydrochemical elucidation of coastal groundwater in and around Kalpakkam region, southern India. Environ Earth Sci 64(5):1419–1431
Chidambaram S, Bala Krishna Prasad M, Manivannan R, Karmegam U, Singaraja C, Anandhan P (2012) Environmental hydrogeochemistry and genesis of fluoride in groundwaters of Dindigul district, Tamilnadu (India). Environ Earth Sci 68(2):333–342
Chidambaram S, Bhandary H, Hadi K (2020) CHIDAM—A software for chemical interpretation of the dissolved ions in aqueous media. Groundw Sustain Dev. https://doi.org/10.1016/j.gsd.2020.100496
Cho BW, Choo CO (2019) Geochemical Behavior of Uranium and Radon in Groundwater of Jurassic Granite Area, Icheon, Middle Korea. Water 11:1278. https://doi.org/10.3390/w11061278
Christopher CF, Bargar JR, JA Davis (2003) Remediation of Uranium-contaminated ground water at fry canyon, Utah. Science Highlight
Cothern CR, Lappenbusch WL (1983) Occurrence of Uranium in drinking water of U.S. Health Phys 45:89–99
Cowart JB, Osmond JK (1977) Uranium isotopes in ground water: their use in prospecting for sandstone-type uranium deposits. J Geochem Explor 8:365–379
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater in Delhi area: chemical weathering processes and groundwater flow regime. J Geol Soc India 47:179–188
De Camargo IMC, Mazzilli B (1996) Determination of uranium and thorium isotopes in mineral spring waters. J Radioan Nucl Chem 212(4):251–258
Dehnavi AG, Sarikhani R, Nagaraju D (2011) Hydro geochemical and rock water interaction studies in East of Kurdistan, N-Wof Iran. Int J Environ Sci Res 1(1):16–22
Dressen DR, Williams JM, Marple ML, Gladney ES, Perrin DR (1982) Mobility and bioavailbility of uranium mill tailings contaminants. Environ Sci Technol 16(10):702–709
Durov SA, Akad Nauk SSSR (1948) Study on uranium in mill tailings. J Environ Radioact 59:87–90
Fiedor JN, Bostick WD, Jarabek RJ, Farrell J (1998) Understanding the mechanism of uranium removal from groundwater by zero-valent iron using X-ray photoelectron spectroscopy environ. Sci Technol 32:1466–1473
Finch RJ, Ewing RC (1992) The corrosion of uraninite under oxidizing conditions. J Nucl Mater 190:133–156
GSI (Geological Survey of India) (1995) Geological and mineral map of Tamil Nadu and Pondicherry, 1:500,000
Gueniot B, Munier Lamy C, Berthelin J (1988) Geochemical behavior of uranium in soils, part II. Distribution of uranium in hydromorphic soils and soil sequences. Applications for surficial prospecting. J Geochem Explor 31(1):39–55
Guthrie VA, Kleeman JD (1986) Changing uranium distribution during weathering of granite. Chem Geol 54(1–2):113–126
Hill RA (1940) Geochemical patterns in Coachella Valley. Trans Am Geophys Union 21:46–49
Hsi C, Langmuir D (1985) Adsorption of uranyl onto ferric oxyhydroxides: application of the surface complexation site-binding model. Geochim Cosm Acta 49:1931–1941
Janeezek J (1991) Composition and origin of coffinite from Jachymov Czechoslovakia. N J b Miner M h 9:385–395
Janeezek J, Ewing RC (1992) Coffinitization: a mechanism for the alteration of UO2 under reducing conditions. Mater Res Soc Symp Proc 257:497–504
Jerden JL, Sinha AK, Zelazny L (2003) Natural immobilization of uranium by phosphate mineralization in an oxidizing saprolite-soil profile: chemical weathering of the Coles Hill uranium deposit, Virginia. Chem Geol 199:129–157
Johnson RW, Mackenzie DE, Smith IE (1970) Short papers on quaternary volcanic areas in Papua New Guinea. Bur Miner Resour Aust Rec 1970/72 (unpublished)
Jordana S, Batista E (2004) Natural groundwater quality and health. Geol Acta Int Earth Sci J 2(2):175–188
Jurgens BC, Fram MS, Belitz K, Burow KR, Landon MK (2009a) Effects of groundwater development on uranium: central Valley, California, USA. Ground Water 49:913–928
Jurgens BC, Fram MS, Belitz K, Burow KR, Landon MK (2009b) Effects of groundwater development on Uranium: central valley, California, USA, USGS Staff-Published Research
Karmegam U (2012) A study on the hydrogeochemical modeling of coastal aquifers in and around Kalpakkam. Unpublished Ph.D Thesis, Department of Earth Sciences, Annamalai University, India
Killiari T, Pashalidis I (2010) Simplified alpha-spectroscopic analysis of uranium in natural waters after its separation by cation-exchange. Radiat Meas 45(8):966–968
Kim K, Rajmohan N, Yun S-T, Kim H-J, Baohua Gu, Kim M-H, Hwang G-S, Lee S-H (2005) Evaluation of geochemical processes affecting groundwater chemistry based on mass balance approach: a case study in Namwon, Korea. Geochem J 39:357–369
Kurttio P, Auvinen A, Salonen L, Saha H, Pekkanen J, Makelainen I, Vaisanen SB, Penttila IM, Komulainen H (2002) Renal effects of uranium in drinking water. Environ Health Perspect 110:337–342
Langmuir D (1997) Aqueous environmental geochemistry. Prentice Hall, Hoboken, p 600
Lowrance R, Altier LS, Newbold JD, Schnabel RR, Groffman PM, Denver JM, Correll DL, Gilliam JW, Robinson JL, Brinsfield RB, Staver KW, Lucas W, Todd AH (1997) Water quality functions of riparian forest buffers in Chesapeake Bay watersheds. Environ Manag 21:687–712
Mahalakshmi M, Gunarani GI, Gnanasekar M, Rajaram S, Sahoo S K (2017) Study of Uranium concentration in groundwater in Nagapattinam District of Tamil Nadu India. J Chem Pharm Sci JCPS 10(1):235–242
Mao Y, Desmeules M, Schaubel D, Berube D, Ducl R, Brule D (1995) Inorganic components of drinking water and microalbuminuria. Environ Res 71:135–140
Meinrath G, Volke P, Dudel EG, Merckel BJ (1999) Determination and interpretation of environmental water samples contaminated by uranium mining activities. Frensenius J Anal Chem 364:191–202
Merkel JB, Planer-Friedrich B (2005) Edited by. Darrell Kirk Nordstrom. Groundwater. Geochemistry. A Practical Guide to Modeling of Natural and Contaminated Aquatic. Systems. Springer, Berlin.
Morrow P, Gelein R, Beiter H, Scott J, Picano J, Yuile C (1982) Inhalation and intravenous studies of UF6/UO2F2 in dogs. Health Phys 43:859–873
Murray FH, Brown JR, Fyfe WS, Kronberg BI (1983) Immobilization of U-Th-Ra in mine wastes by phosphate mineralization. Can Mineral 21:607–610
NRC (1983) Preparation of environmental reports for uranium mills: regulatory guide 3.8-October 1982, Rev 2. http://pbadupws.nrc.gov/docs/ML0037/ML003740211.pdf
Nur A, Ayuni KN (2011) Hydrogeophysical study of Michika and environs, northeast Nigeria. Int J Phys Sci 6(34):7816–7827
Osmond JK, Cowart JB (1976) The theory and uses of natural uranium isotopic variations in hydrology. At Energy Rev 14(4):621–679
Pandey K (1995) Uranium and thorium abundances in some graphite-bearing Precambrian rocks of India and implications. Res Commun Curr Sci 68(8):826–828
Paramaguru P, Anandhan P, Chidambaram S, Ganesh N, Nepolian M, Devaraj N, Vasudevan U, Rakesh RG, Pradeep K (2016) Appraisal of groundwater quality in the Cuddalore district of Tamilnadu. Int Res J Earth Sci 4(6):23–30
Parkhurst DL, Appelo CAJ (1999) User's guide to PHREEQC (version 2): a computer 524 program for speciation, batch-reaction, one-dimensional transport, and inverse 525 geochemical calculations. U.S. Geological Survey, Denver, Colorado
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–923
Plummer LN, Busby JF, Lee RW, Hanshaw BB (1990) Geochemical modeling of the Madison aquifer in parts of Montana, Wyoming, and south Dakota. Water Resour Res 26:1981–2014
Prasanna MV, Chidambaram S, Shahul Hameed A, Srinivasamoorthy K (2010) Study of evaluation of groundwater in Gadilam basin using hydrogeochemical and isotope data. Environ Monit Assess 168:63–90
Prasanna MV, Chidambaram S, Shahul Hameed A, Srinivasamoorthy K (2011) Hydrogeochemical analysis and evaluation of groundwater quality in the Gadilam river basin Tamilnadu. J Earth Sci 120(1):85–98
Rajesh K, Jha VN, Sahoo SK, Jha S, Kumar S, Ravi PM, Tripathi RM (2015) Distribution of naturally occurring radionuclides uranium and Ra in groundwater adjoining uranium complex of Turamdih, Jharkhand, India. Curr Sci 108:12
Ramesh R, Anbu M (1996) Chemical methods for environmental analysis-water and sediments. p 161
Reynolds BC, Wasserburg GJ, Baskaran M (2003) The transport of U and Th-series nuclides in sandy confined aquifers. Geochim Cosmochim Acta 67(11):1955–1972
Selvi B, Vijayakumar B, Rana B, Ravi P (2016) Distribution of natural uranium in groundwater around Kudankulam. Radiat Protect Environ 39(1):25
Senior LA (1998) Radon-222 in the ground water of Chester County, Pennsylvania. In: Water-resources investigations report 98-4169. U.S. Geological Survey
Shabansefidan F, Anji Reddy M (2012) Investigation of Uranium solubility and its transport path in the Razgah Basin’s groundwater in the Northwest of Iran Using Saturation Indexes. IJCSMS 4:39–43
Siegel MD, Bryan CR (2004) Environmental geochemistry of radioactive contamination, treatise on geochemistry, p 262
Sowder AG (1998) The formation, transformation, and stability of environmentally relevant Uranyl mineral phases. Ph.D. Dissertation, Clemson University
Sprinkle CR (1989) Geochemistry of the Floridan aquifer system in Florida and in parts of Georgia, South Carolina, and Alabama: U.S. Geological Survey Professional paper 1403-I, p 105
Srinivasamoorthy K, Chidambaram S, Prasanna MV, Vasanthavigar M, John Peter A, Anandhan P (2008) Identification of major sources controlling groundwater chemistry from a hard rock terrain—A case study from Mettur taluk, Salem district, Tamilnadu India. J Earth Syst Sci 117(1):49–58
Srinivasamoorthy K, Vasanthavigar M, Vijayaraghavan K, Chidambaram S, Anandhan P, Manivannan R (2011) Use of hydrochemistry and stable isotopes as tools for groundwater evolution and contamination investigations. Geoscience 1(1):16–25
Sumner ME (2000) Soil fertility and plant nutrition. In: Section D in handbook of soil science. CRC Press, Boca Raton
Thilagavathi R, Chidambaram S, Thivya C, Prasanna MV, Singaraja C, Tirumalesh K, Pethaperumal S (2014) Delineation of natural and anthropogenic process controlling hydrogeochemistry of layered aquifer sequence. Proc Natl Acad Sci 84(1):95–108
Thivya C (2014) Study on Uranium in groundwater of Madurai district, Tamilnadu (unpublished Ph.D. thesis). Department of Earth Sciences, Annamalai University, Tamilnadu, India
Thivya C, Chidambaram S, Thilagavathi R, Prasanna MV, Singaraja C, Nepolian M, Sundarrajan M (2013a) Identification of the geochemical processes in groundwater by factor analysis in hard rock aquifers of Madurai district, South India. Arab J Geosci 7:3767–3777
Thivya C, Chidambaram S, Singaraja C, Thilagavathi R, Prasanna MV, Jainab I (2013b) A study on the significance of lithology in groundwater quality of Madurai district, Tamil Nadu (India). Environ Dev Sustain 15:1365–1387
Thivya C, Chidambaram S, Tirumalesh K, Prasanna MV, Thilagavathi R, Nepolian M (2014) Occurrence of the radionuclides in groundwater of crystalline hard rock regions of central Tamil Nadu, India. J Radio Anal Nucl Chem 302:1349–1355
Thivya C, Chidambaram S, Tirumalesh K, Prasanna MV, Thilagavathi R, Adithya VS, Singaraja C (2015a) Lithological and hydrochemical controls on distribution and speciation of uranium in groundwaters of hard-rock granitic aquifers of Madurai District, Tamil Nadu (India). Environ Geochem Health 37(3)
Thivya C, Chidambaram S, Thilagavathi R, Tirumalesh K, Rao MS, Prasanna MV, Nepolian M (2015b) Occurrence of high uranium and radon in hard rock aquifers of south India-evaluating the temporal and spatial trends. Groundw Sustain Dev 1:68–77
Thunqvist E (2004) Regional increase of mean chloride concentration in water due to the application of deicing salt. Sci Total Environ 325:29–37
Tieh TT, Ledger EB, Rowe MW (1980) Release of uranium from granitic rocks during in situ weathering and initial erosion (Central Texas). Chem Geol 29:227–248
Toulhoat P, Beaucaire C (1991) Comparison between lead isotopes 234U/238U activity ratio and saturation index in hydrogeochemical exploration for concealed uranium deposits. J Geochem Explor 41:181–196
Trusdell AH, Jones BF (1973) WATEQ: A computer program for calculating chemical equlibria of natural waters. J Res US Geol Surv 2(2):233–248
Van der Lee J (1998) Thermodynamic and mathematical concepts of CHESS. Ecole des 567 Mines de Paris: Fontainebleau, France
Westall J, Zachary J (1986) MINEQL: A computer program for the calculation of the chemical equilibrium composition of aqueous systems. Department of chemistry, Oregon State University
WHO (World Health Organization) (2012) Guidelines for drinking water quality-4th edition. ISBN 9789241548151 (NLM Classifcation: WA 675). World health Organization, Geneva
Wilding LP, Smeck NE, Drees LR (1977) Silica in soils: quartz, cristobalite, tridymite, and opal. In: Dixon JB, Weed SB (eds) Minerals in soil environments, Chapter 14. Soil Science Society of America, Madison
Wrenn MD, Singh NP (1982) Comparative distribution of uranium, thorium, and plutonium in human tissues of the general population. In: Vohra KC (ed) Natural radiation environment. Wiley Eastern Ltd, New Delhi, pp 144–154
Yidana SM, Ophori D, Banoeng-Yakubo B (2008) A multivariate statistical analysis of surface water chemistry: the Ankobra Basin, Ghana. Environ Manag 86:80–87
Zamora ML, Tracy BL, Zielinski JM (1998) Chronic ingestion of uranium in drinking water: a study of kidney bioeffects in humans. Toxicol Sci 43:68–77
Zapecza OS, Szabo Z (1986) Natural radioactivity in ground water- a review. In: U.S. Geological Survey National Water Summary 1986, ground-water quality: hydrologic conditions and events. U.S. Geological Survey Water Supply Paper 2325, pp 50–57
Funding
This study was financially supported by the University Grants Commission (UGC) No. F: 39-143/2010 (SR) dated December 27, 2010.
Author information
Authors and Affiliations
Contributions
All authors listed on the title page have contributed significantly to the work, have read the manuscript, attest to the validity and legitimacy of the data and its interpretation, and agree to its submission.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies involving animals and human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chandrasekar, T., Sabarathinam, C., Viswanathan, P.M. et al. Potential interplay of Uranium with geochemical variables and mineral saturation states in groundwater. Appl Water Sci 11, 75 (2021). https://doi.org/10.1007/s13201-021-01396-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01396-3