Abstract
This study investigates the hydrogeochemical and anthropogenic factors that control groundwater quality in an Upper Precambrian sedimentary aquifer in the northwestern Burkina Faso. The raw data and statistical and geochemical modeling results were used to identify the sources of major ions in dug well, private borewell and tap water samples. Tap waters were classified as Ca–HCO3 and Ca–Mg–HCO3 types, reflecting the weathering of the local dolomitic limestones and silicate minerals. Dug well waters, with a direct contact with various sources of contamination, were classified as Ca–Na–K–HCO3 type. Two factors that explain 94% of the total variance suggested that water–rock interaction was the most important factor controlling the groundwater chemistry. Factor 1 had high loadings on pH, Ca2+, Mg2+, HCO3−, SO42− and TDS. These variables were also strongly correlated indicating their common geogenic sources. Based on the HCO3−/(HCO3− + SO42−) ratios (0.8–0.99), carbonic acid weathering appeared to control Ca2+, Mg2+, HCO3− and SO42− acquisition in the groundwater. With relatively lower Ca2+ and Mg2+ concentrations, the majority of dug well and borewell waters were soft to moderately hard, whereas tap waters were considered very hard. Thus, the dug well and, to a lesser extent, borewell waters are likely to have a low buffering capacity. Factor 2 had high loadings on Na+, NO3− and Cl−. The strong correlation between Na+ and NO3− and Cl− implied that factor 2 represented the anthropogenic contribution to the groundwater chemistry. In contrast, K+ had moderate loadings on factors 1 and 2, consistent with its geogenic and anthropogenic sources. The study demonstrated that waters from dug wells and borewells were bacteriologically unsafe for human consumption, and their low buffering capacity may favor mobility of potentially toxic heavy metals in the aquifer. Not only very hard tap waters have aesthetic inconvenient, but their consumption may also pose health problems.
Similar content being viewed by others
Introduction
Following severe droughts in 1970s, a massive internal migration from drier central plateau and northern regions toward a more humid northwestern Burkina Faso has put a tremendous pressure on the regional surface water resources (Kessler and Greerling 1994). The northwestern Burkina Faso has been also subject to adverse effects of climate changes such as erratic precipitations and decrease in seasonal surface water flow, and thus, surface water becomes an unreliable source for water supply. As a result, people have been heavily relying on groundwater for domestic water supply and livestock watering (Derouane and Dakoure 2006; Courtois et al. 2010; Huneau et al. 2011). Traditional hand-dug wells are the main sources of groundwater in the region. In order to meet the ever-increasing demands for water, hundreds of borewells, equipped with hand pumps, were drilled in the Kossi Province one of the four provinces in the northwestern Burkina Faso (Barry et al. 2005) and the site of the present study. The borewells draw groundwater from deep fractured sedimentary rocks, whereas the dug wells abstract shallow groundwater within weathered mantle layers (Collectif 1990).
Although groundwater constitutes an important asset for socioeconomic development of the northwestern Burkina Faso, the hydrogeochemical studies pertaining groundwater quality in this large transboundary aquifer are scanty. The local groundwater quality is likely to be controlled by both natural and anthropogenic factors. Water–rock interaction (i.e., chemical weathering and cation exchange processes) can be the most important natural factor that controls the groundwater quality (Fetter 1994; Appelo and Postma 2005; Li et al. 2016). In contrast, excessive use of fertilizer, non-protection of wells and poor sanitary conditions are potential sources of anthropogenic pollution (Groen et al. 1988; Li et al. 2017; Yameogo and Savadogo 2002; Huneau et al. 2011; Wu et al. 2017). The monitoring of the physicochemical and biological conditions of groundwater is necessary for an efficient water resource management and development of aquifer protection strategies. Therefore, the objectives of the present study were (1) to identify the hydrogeochemical processes and anthropogenic activities that govern the chemical composition of dug wells, private borewells and tap water provided by the public water supply system of an Upper Precambrian sedimentary aquifer, and (2) to evaluate the suitability of the groundwater for human consumption. The findings of this study will contribute to bridging the gap between anthropogenic factors and hydrogeochemical processes that control groundwater quality in a sedimentary and semi-urban setting.
Site description
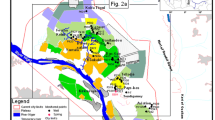
The study area is located in the town of Nouna, the Kossi Province (Northwestern), 306 km of Ouagadougou the capital city of Burkina Faso (Fig. 1a). The area is part of a floodplain of the ephemeral Kossi River basin (Fig. 1b). This plain contains several ponds of variable sizes, separated by elevated zones (200–300 m a.s.l). The local climate is of the north-Sudanian type, characterized by a dry season (October–May) and a wet season (June–September). With an average annual rainfall of 887 mm, the Nouna commune falls in the so-called the Bread Basket of Burkina Faso, where subsistence and cotton farming and livestock bring a substantial income to the populations. As in the whole country, the plain has undergone a marked decrease in rainfall since the 1970s (~ 200 mm), putting a great pressure on water resources. Currently, rainfall is characterized by a great intra- and inter-annual irregularity (Frappart et al. 2009).
a Geographical map of Burkina Faso; b geomorphological map of the Kossi floodplain, showing the study area; c groundwater sampling points superimposed on the simplified local lithological units. The lithology of tap water from the public water supply points may not correspond to their sampling lithology
The area is underlain by Upper Precambrian sedimentary rocks known as the southeast Taoudeni sedimentary formations shared by Mali and Burkina Faso. These formations are essentially made of an alternation of pink siltstones and argillites with glauconite and dolomitic limestone lenses capped with silexite (Ouédraogo 1998). As in the crystalline basement areas that make up 80% of Burkina Faso, two types of discontinuous aquifers are encountered in the study area. A shallow (5–20 m) aquifer located in the weathered lateritic layer, which is superimposed on a deep aquifer within the joined sandstone layers in the sedimentary sequence (CIEH 1976; BILAN D’EAU 1993). The thickness of the deep aquifer is poorly known, and it varies according to the lithology. In contrast to crystalline basement aquifers, the high permeability (1.8 × 106 m/s) of sedimentary rocks makes the southeast Taoudeni sedimentary formations excellent aquifers, with an estimated storage coefficient of 1 × 10−4 and significant yields up to 100 m3/h (Gombert 1998). That is, the only two permanent watercourses in the country (i.e., the Mouhoun and Comoé rivers) are directly fed by springs originated from sedimentary aquifers (Talbaoui 2009).
The local groundwater recharge occurs through direct infiltration of rainwater and indirect infiltration of runoff via depressions, streams and alluvial valleys (Groen et al. 1988; Barry et al. 2005). The regional water table shows a seasonal variation of 1–2 m. The estimated total volume of groundwater in the Nouna commune is 0.4 million m3/year, whereas the renewable resource is about 0.5 million m3/year (MEE 2001). Consequently, groundwater resource development in the commune is very limited compared to the resource availability. More than half of the resources are used for domestic water supply and the remaining for livestock watering (MEE 2001). Poor sanitation, lack of an effective management of domestic wastes, inadequate protection of dug wells from surface runoff and animal droppings make the groundwater highly vulnerable to anthropogenic pollution.
Materials and methods
Twenty groundwater samples were collected from six major wards of Nouna in dry season 2017 (Fig. 1c). Five samples were collected from representative private borewells (B1–B5), five from shallow hand-dug wells with large diameters (W1–W2), whereas 10 samples were collected from the public water supply system (P1–P10; Table 1). In order to obtain high water flow rates, the groundwater supplied by the public water supply system is abstracted from relatively deeper aquifers. The hand-dug well samples were drawn using a sterilized bucket and filtered through Millipore membrane (0.45 µm) into two sets of new high-density polyethylene bottles (HDP), whereas those of borewells and tap water were directly pumped through filter capsules into two sets of HDP. One set of the samples was acidified with ultrapure HNO3− (pH > 3), whereas the other set was left non-acidified. A third set of samples was collected and kept unfiltered and non-acidified in glass bottles for bacteriological counts. Electrical conductivity (EC), pH and total dissolved solids (TDS) were measured in the field using calibrated meters with standard solutions. The samples were put in ice box and taken to laboratory for major cation and anion analysis.
In the laboratory, concentrations of Ca2+ and Mg2+ were estimated titrimetrically using 0.05 N EDTA and 0.01 N, whereas those of HCO3− and Cl− by H2SO4 and AgNO3 titration, respectively. Sodium and K+ concentrations were determined by flame photometric method (APHA 1995), and those of SO42− and NO3− by UV–Vis spectrophotometric technique. Total hardness (TH) was determined by EDTA complexometric titration method (WHO 1999). Analytical reagent grades and milli-Q water were used for the analyses. Two borewell samples (B1 and B2) had large charge balance errors (> ± 10%) and were not included in the data interpretation.
Nutrient MacConkey agar was used for total coliform bacterial count and Eosin for total fecal coliform. The petri dishes containing agar and diluted groundwater samples were incubated under appropriate conditions (time and temperature). The bacteriological counts per 100 mL were estimated from the MPN table (APHA9221D).
R-mode factor analysis (Wu et al. 2014) was used to assess the relationships between the physicochemical parameters of the groundwater, using SPSS package (version 20), whereas Visual MINTEQ (version 3.1) was used to calculate saturation indices (SI) of carbonate and evaporite minerals as well as partial CO2 pressure of the groundwater.
Results and discussion
Groundwater constituents
The physicochemical data of groundwater highlighted distinct differences between shallow dug well, borewell and tap waters. A strong relationship (R2 = 0.96) between total cations TZ+ and total anions TZ− (Fig. 2a) implied that contribution of non-measured ions to charge balance was not significant. Furthermore, the relationship between EC and TDS (R2 = 0.96; Fig. 2b) suggested that the groundwaters were unlikely to contain substantial amounts of uncharged soluble compounds (e.g., silica, manganese, aluminum and iron) that may contribute to TDS contents (Datta and Tyagi 1996; Prasanna et al. 2011).
In overall, EC and TDS were low in the groundwaters (Table 1). This suggests the absence of salt in the recharge water and limited groundwater mineralization (Han and Liu 2004; Smedley et al. 2007; Huneau et al. 2011; Jeannin et al. 2016). Because of intense leaching, groundwaters drawn from the weathered mantle aquifer appeared to be less mineralized compared to those from the deep fractured aquifer. As result, ZT+ and TZ− were higher in tap waters (medians = 172 and 336 µeq/L; Table 2) from the deep aquifer than in dug wells from the weathered mantle aquifer. The differences in recharge flow paths could also be an explanation for the observed mineralization trends. Thus, weakly mineralized groundwaters are often associated with rapid recharge (i.e., younger residence time) of the shallow aquifers, whereas highly mineralized groundwaters (i.e., older residence time) have been attributed to paleo-recharge or slow circulation processes in deep aquifers (Fritz 1997; Stober and Bucher 1999; Cook et al. 2005; Bucher and Stober 2010; Armandine Les Lands et al. 2014).
The high coefficients of variance (CV > 50%; Table 2) and spatial distribution, illustrated by boxplots (Fig. 3), showed a heterogeneous abundance of most physicochemical parameters in dug wells. This is probably due to the sources and the nature of the recharge, the host rock geology, and the short residence time of the groundwater in the weathered mantle aquifer (Back and Hanshaw 1971). On contrary, groundwater composition of tap waters was remarkably homogeneous (CV < 50%; except Na+) and most variables had similar values for mean and median, reflecting primarily the long flow lines and dispersive mixing that may have smoothed out any temporal fluctuations in the groundwater composition (Mazor et al. 1993; Dhar et al. 2008). Although the majority of the samples had pH values within the World Health Organization (WHO 2006) guideline limit for drinking water (pH = 6.5–8.5), the dug well and borewell waters had lower pH (medians = 6.2 ± 0.2 and 6.4 ± 0.9) relative to those of tap waters (median = 7.8 ± 0.2). The high pH in tap waters relative to dug well waters is consistent with positive correlations between pH and the resident time usually observed in deeper aquifers (Morgenstern and Daughney 2012). Total hardness (TH) in the well waters had distribution patterns similar to those of pH, TDS and EC with TH ranging from 33 to 236 mg CaCO3/L. The dug well waters exhibited the lowest TH (median = 47 ± 24.6 mg CaCO3/L and CV = 44%), whereas the highest concentrations were observed in tap waters (median = 258 ± 18 mg CaCO3/L and CV = 18%).
a Boxplots of naturally affected physicochemical parameters in the Upper Precambrian sedimentary aquifer of the northwestern Burkina Faso. The tops and bottoms of the boxes represent the 75th and 25th percentiles, respectively. The horizontal line across the boxes indicates the median. The vertical lines from the tops and bottoms of the boxes extend to 90th and 10th percentiles, respectively. b Boxplots of anthropogenically affected physicochemical parameters in the Upper Precambrian sedimentary aquifer of the northwestern Burkina Faso
Again, the high TH in tap waters can be attributed to long residence time of groundwater in the deep fractured aquifer, leading to extended chemical weathering of dolomitic limestones (Frape et al. 1984). With hardness values largely exceeding the WHO guideline value for drinking water, the tap waters were categorized as very hard, while those of dug wells as soft to moderately hard. Soft waters, with low alkalinity and buffering capacity, may favor the mobility of potentially toxic heavy metals in the aquifer (De Schamphelaere and Janssen 2004; Kirby and Cravotta 2005). In contrast, hard waters require more soap to produce lather, and thus, it is unsuitable for domestic use (Srinivasa Rao and Jugran 2003). Some evidence has also indicated the role played by hard waters in heart diseases and prenatal mortality (Schroeder 1960; Agarwal and Jagetai 1997). Although such cases have not been reported in the present study area, the desirability of softer drinking water is evident among the local population. As a result, the water provided by the public water supply system should be treated before it gets to the consumers.
Sodium was the dominant cation in dug well waters followed by Ca2+, K+ and Mg2+, whereas cation abundance in borewell and tap waters was in decreasing order of Ca2+> Mg2+> K+>Na+ (Table 1). The low EC, TDS, HCO3− and TH contents observed in dug well and borewell waters suggest short contact times between groundwater and the aquifer minerals. This is consistent with the low K+ (except W5 and B4) concentrations in dug well and borewell waters relative to tap waters (8–11 mg/L). Potassium concentrations in groundwater up to 10 mg/L are attributed to orthoclase or clay weathering, whereas concentrations above 10 mg/L may indicate external sources of K+ abundance (Rail 2000). Bicarbonate, SO42− and NO3− were the dominant anions in the wells with the highest HCO3− and SO42− concentrations observed in tap waters. Although these ion concentrations in the groundwater were within the WHO permissible limits for drinking water, NO3− concentrations in dug well waters exceeded the natural nitrate concentrations (5–7 mg/L; Appelo and Postma 1999). None of dug well and borewell samples complied with the WHO guideline values for coliforms (Table 1), and hence, water from these wells requires treatment before human consumption.
Processes controlling groundwater chemistry
Chemical weathering, cation exchange, evaporation and antropogenical activities are the common hydrogeochemical processes that control groundwater chemistry. In order to shed light on these complex processes, statistical and geochemical techniques were used. Thus, the R-mode factor analysis, after varimax rotation (Kaiser 1960), produced two factors (with eigenvalues > 1) that explain 94% of the total variance (Fig. 4). With 63.4% of the total variance, factor 1 is the most important factor that influences the groundwater chemistry. This factor had high absolute loadings on Ca2+, Mg2+, TH, HCO3−, pH, SO 2−,4 EC and TDS and a moderate loading on K+. As expected, there were strong positive correlations between Mg2+ and Ca2+ (r = 0.96) and between TH and Ca2+ and Mg2+ (r = 097 and 0.99, respectively). The pH was also positively correlated with TH, Ca2+, Mg2+ and HCO3− (Table 3). That is, an increase in Ca2+, Mg2+ and HCO3− concentrations through chemical weathering will increase the groundwater pH. Therefore, it can be suggested that the factor 1 reflects water–rock interaction within the aquifer.
The influence of water–rock interaction on the groundwater chemistry was examined through bivariate mixing plots of Na+-normalized Ca2+ versus Na+-normalized Mg2+ and Na+-normalized HCO3− on log–log scale (Fig. 5; Gaillardet et al. 1999). Gaillardet et al. (1999) used published data of well-characterized lithologies to determine silicate and carbonate end members. According to these authors, the carbonate end member is characterized by Ca/Na, Mg/Na and HCO3/Na ratios of 45 ± 25, 15 ± 10 and 90 ± 40 mg/L, respectively, whereas the chemistry of water draining silicate is characterized by Ca/Na = 0.3 ± 0.15 mg/L, Mg/Na = 0.24 ± 0.12 mg/L and HCO3/Na = 2 ± 1 mg/L. In the present study, the bivariate plots identified silicate weathering and carbonate dissolution as the two hydrogeochemical processes controlling the groundwater chemistry. Dug well and, to a lesser degree, borewell samples plotted closer to the silicate end member, while those of tap water tended toward the carbonate end member (Fig. 5a, b). Because of their proximity to the surface, dug well waters were closer to the evaporite dissolution end member than those of borewells and tap waters (Fig. 5).
The extent of water–rock interaction was further assessed through the molar ratios of Mg2+/Ca2+ of the samples. All samples had Mg2+/Ca2+ ratios less than 2 (Table 4), indicating silicate weathering (Weaver et al. 1995). The average molar ratios of (Ca2+ + Mg2+)/TZ+ (0.44 and 0.48) in the borewells also exceeded those of tap waters (Na+ + K+)/TZ+ (0.13 and 0.04). This reflects weathering of dolomitic limestones in the source aquifer. In contrast, the average (Na+ + K+)/TZ+ ratio was slightly higher than that of (Ca2+ + Mg2+)/TZ+ in dug wells. Thus, the behavior of alkali and alkaline earth ions in the dug wells may be controlled by cation exchange between the groundwater and the clay minerals often encountered in the lateritic layers. The chloro-alkaline indices (CAI-1 and CAI-2) were used to study a possible ion exchange between the groundwater and the aquifer materials during the residence time and movement (Schoeller 1965; Marghade et al. 2012). The chloro-alkaline indices (all the ions are expressed in meq/L) were calculated as follows (Eqs. 1, 2):
If both CAI-1 and CAI-2 are negative, Ca2+ and Mg2+ have been adsorbed onto the aquifer materials and Na+ or/and K+ are released in the groundwater (i.e., reverse ion exchange). In contrast, if the indices are positive, alkaline earth ions (Ca2+ and Mg2+) have been released in the groundwater and alkalis retained by the aquifer materials (i.e., direct ion exchange; Schoeller 1967). The Schoeller indices of the groundwater samples of the present study were negative (Fig. 6a), suggesting that reverse ion exchange could contribute to Na+ and K+ abundance in the wells. However, the linear plot (Fig. 6b) between Na+ + K+–Cl− and (Ca2+ + Mg2+)–(SO42− + HCO3−) showed a weak relationship (R2 = 0.132) and a slope of 1.214. This is far from the theoretical correlation (R2 > 90%) coefficient and slope of about − 1 (Fisher and Mullican 1997; Wen et al. 2005; Yidana and Yidana 2010). Therefore, it can be assumed that chemical weathering is the single most important hydrogeochemical process that controls distribution of Ca2+, Mg2+, SO42− and HCO3− in the groundwaters. The abundance of these ions in the groundwaters is a function of carbonate mineral distribution in the host aquifer materials.
Thus, the relative high Ca2+, Mg2+, SO42− and HCO3− concentrations in tap waters corroborates the availability of carbonate minerals in deeper aquifers as well as longer residence times of the groundwater. As a result, samples from tap water were saturated with carbonate minerals (Table 4). Nevertheless, the calcite saturation indices did not correlate with TDS, Ca2+ and HCO3− suggesting that calcite did not continue to dissolve in the aquifer following its saturation (Fig. 7). In contrast, strong linear relationships existed between Ca2+ (R2 = 0.76), TDS (R2 = 0.61), Mg2+ (R2 = 0.78) and HCO3− (R2 = 0.73) and dolomite saturation indices. Similarly, gypsum saturation indices correlated well with TDS (R2 = 0.62; Fig. 7). This indicates that the groundwaters have the capacity to dissolve dolomite and gypsum, and the bulk of Ca2+, Mg2+ and SO42− concentrations is assumed to be from dissolution of these minerals.
Only moderate positive correlations were observed between K+, pH, TDS, Ca2+ and Mg2+, which suggested that K+ were only partially influenced by chemical weathering. In addition to orthoclase dissolution, excessive application of KCl as a fertilizer may have contributed to K+ and Cl− loadings in the groundwater (Lee et al. 2005). Because the groundwaters were under-saturated with respect to gypsum, the low SO −24 concentrations, particularly in dug wells (SO4/Cl > 1), indicated a possible sulfate reduction by microorganisms (Lavitt et al. 1977; Datta and Tyagi 1996). Further evidence to the microbial activities is highlighted by high bacterial counts and high partial pressures of CO2 (pCO2) in the dug wells (Tables 1, 4). That is, the calculated pCO2 of the groundwater were greater than that of the atmospheric pCO2 (10−3.4 atm) with the highest values observed in the dug wells. This suggests that infiltrating water into the aquifer via soil tends to have higher dissolved CO2 produced by organic matter decomposition and root respiration (Eq. 3). This biogeochemical process is likely to produce carbonic acid (H2CO3) in the groundwater (Eq. 4), which is responsible for mineral weathering (Eqs. 5, 6; Drever 1988).
The substantial decline in pCO2 followed by an increase in pH in tap water could be attributed to CO2 outgassing in deep aquifers (Subba et al. 2006). Another source of proton in the groundwater could be sulfide mineral oxidation (Eq. 7; Berner and Berner 1987; Sarin et al. 1989; Singh and Hasnain 2002).
Carbonic acid and sulfide mineral oxidation weathering can be distinguished by the HCO3−/(HCO3− + SO42−) ratios (Pandey et al. 2001). The HCO3−/(HCO3− + SO42−) ratio equal to 1 indicates that carbonic acid is the main proton source for chemical weathering, whereas a ratio of 0.5 suggests that both carbonic acid and the proton from pyrite oxidation were responsible for the groundwater ion acquisition. In the present groundwater samples, HCO3−/(HO3− + SO42−) varied from 0.8 to 0.99, suggesting that carbonic acid weathering of carbonate, dolomite and gypsum controlled the abundance of Ca2+, Mg2+, HCO3− and SO42− in the groundwater.
Factor 2 had high loadings on Na+, Cl− and NO3−. Although Na+ may derived from silicate weathering (Meybeck 1987), halite dissolution, a strong positive correlation between Na+ and NO3−, an index of anthropogenic activities (David and Gentry 2000), implied that anthropogenic sources such as untreated sewage effluent had greatly contributed to Na+ loading into the groundwater system (Patterson 1997). According to Patterson (1997), laundry detergent powders provide up to 40% of Na+ in wastewater. The anthropogenic contribution to Na+ loading is further corroborated by its relative high concentrations in dug well waters, directly influenced by surface pollution, compared to tap waters from deeper aquifer. The strong relationship observed between Na+ and Cl− (r = 0.94) could be attributed to halite dissolution as all samples were under-saturated with respect to halite. However, if there were halite deposits within the aquifer sediments, one could expect to find localized saline waters (high TDS) in the groundwater. Instead, dug well waters, with relatively low TDS, exhibited the highest Cl− concentrations. Halite dissolution cannot therefore be the main source of Cl− in the groundwater. Furthermore, Cl concentration in rock-forming minerals (biotite) commonly found in the study area is thought to be very low, and that weathering is unlikely to be the source of Cl− in the groundwater. Atmospheric deposition (dust and rainfall) and decomposition of organic matter may be the primarily source of Cl− abundance in the present groundwater (Freeze and Cherry 1979). The atmospheric origin of Cl− was further supported by the low Cl/TZ− (< 1) of the groundwater, and hence, Cl− would be present as NaCl (Kortatsi et al. 2008). Thus, factor 2 reflects anthropogenic influence on the groundwater quality.
A moderate positive correlation between K+ and Cl− (r = 0.53) and between and Na+ (r = 0.49) implied that both geogenic and anthropogenic sources had contributed to K+ loading in the groundwater. Based on the water–rock interaction types, Piper triplot (Piper 1944; Fig. 8a) classified tap waters and the majority of borewell waters as Ca–HCO3 or Ca–Mg–HCO3 type, consistent with dissolution of dolomitic limestone and silicate (i.e., amphiboles, pyroxenes, olivine and biotite) minerals. The groundwaters from dug wells were characterized by weathering of aluminosilicate minerals and human activities (Ca–Na–K–HCO3). Furthermore, the Schoeller semi-logarithmic diagram (Schoeller 1962; Fig. 8b) discriminated samples with similar distribution patterns. With longer water–rock interaction, tap waters had higher Mg2+ and Ca2+, SO42− and HCO3− concentrations relative to dug well and borewell waters.
a Piper diagram displaying the dominant water types of the groundwaters; b Schöeller diagram showing major ion distribution patterns of the groundwaters. Tap waters are enriched in Mg2+ and Ca2+, while dug well waters tend to have high Na+ and K+ content. Bicarbonate is the dominant anion in the samples
Conclusions
Factor analysis techniques combined with geochemical modeling successfully identified the natural and anthropogenic factors affecting the groundwater quality in the Nouna sedimentary aquifer. Water–rock interaction (chemical weathering) is the major geochemical process that controls the groundwater chemistry followed by anthropogenic activities. Although all the ions had concentrations within the WHO permissible limits, NO3−, Cl− and, to a lesser degree, K+ were mainly derived from anthropogenic sources. The extent of the Cl− and K+ contamination was pronounced in the dug wells. Due to longer residence times and prolonged water–rock interaction in deeper aquifers, waters supplied by the public water supply system were very hard, whereas those of dug wells and borewells were soft to moderately hard. All dug well samples tested positive for coliform, and thus, they were not suitable for human consumption. In addition to urgent need to improve the general sanitation conditions in Nouna, the dug wells require special care so that the pollutants from various sources can be stopped. Future investigation that includes seasonal variations and heavy metal concentrations is planned.
References
Agarwal V, Jagetai M (1997) Hydrochemical assessment of groundwater quality in Udaipur city, Rajasthan, India. In: Proceedings of national conference on dimensions of environmental stress in India. Department of geology, MS University, Baroda, India, 1997, pp 151–154
APHA (1995) Standard methods for examination of water and waste water, 19th edn. APHA, Washington DC
Appelo CAJ, Postma D (1999) Geochemistry, groundwater and pollution. Balkema, Rotterdam
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. Balkema, Rotterdam
Armandine Les Landes A, Aquilina L, Davy P, Vergnaud V, le Carlier C (2014) Time scales of regional circulation of saline fluids in continental aquifers (Armorican massif, Western France). Hydrol Earth Syst Sci Discuss 11:6599–6635. https://doi.org/10.5194/hess-19-1413-2015
Back W, Hanshaw BB (1971) Geochemical interpretations of groundwater flow systems. W. C. Bull 7:1008–1016. https://doi.org/10.1111/j.1752-1688.1971.tb05021.x
Barry B, Obuobie E, Andreini M, Andah W, Pluquet M (2005) The Volta river basin. Comprehensive assessment of water management in agriculture. International Water Management Institute
Berner EK, Berner RA (1987) The global water cycle: geochemistry and environment. Prentice Hall, Englewood Cliffs
BILAN D’EAU (1993) Carte Hydrogeologique du Burkina Faso
Bucher K, Stober I (2010) Fluids in the upper continental crust. Geofluids 10:241–253. https://doi.org/10.1111/j.1468-8123.2010.00279.x
CIEH (Comité Interafricain d’études hydrauliques) (1976) Carte de planification des ressources en eau souterraine: L’Afrique Soudano-Sahélienne
Collectif (1990) L’hydrogéologie de l’Afrique de l’Ouest. Socle cristallin et cristallophyllien et sédimentaire ancien. Collection Maîtrise de l’Eau. Ministère français du développement et de la coopération, Paris, p 147
Cook P, Love A, Robinson N, Simmons C (2005) Groundwater ages in fractured rock aquifers. J Hydrol 308:284–301. https://doi.org/10.1016/j.jhydrol.2004.11.005
Courtois N, Lachassagne P, Wyns R, Blanchin R, Bougaïré FD, Somé S, Tapsoba A (2010) Large-scale mapping of hard-rock aquifer properties applied to Burkina Faso. Groundwater 48:269–283. https://doi.org/10.1111/j.1745-6584.2009.00620.x
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater in Delhi area: chemical weathering processes and groundwater flow regime. J Geol Soc India 47:179–188
David MD, Gentry LE (2000) Anthropogenic inputs of nitrogen and phosphorus and riverine export for Illinois, USA. J Environ Qual 29:494–508. https://doi.org/10.2134/jeq2000.00472425002900020018x
De Schamphelaere KAC, Janssen CR (2004) Effects of dissolved organic matter concentration and source, pH and water hardness on chronic toxicity of copper to Daphnia magna. Environ Toxicol Chem 23:1115–1122
Derouane J, Dakoure D (2006) Etude hydrogéologique et modélisation mathématique du système aquifère du bassin sédimentaire de Taoudeni au Burkina Faso. Colloque international—Gestion des grands aquifères—30 mai–1er juin, Dijon, France
Dhar RK, Zheng Y, Stute M, van Geen A, Cheng Z, Shanewaz M, Shamsudduha M, Hoque MA, Rahman MW, Ahmed KM (2008) Temporal variability of groundwater chemistry in shallow and deep aquifers of Araihazar, Bangladesh. J Contam Hydrol 99:97–111. https://doi.org/10.1016/j.jconhyd.2008.03.007
Drever JI (1988) The geochemistry of natural waters. Prentice Hall, Englewood Cliffs
Fetter CW (1994) Applied hydrogeology, 3rd edn. Macmillan College, New York, p 616
Fisher SR, Mullican WF (1997) Hydrogeochemical evaluation of sodium-sulphate and sodium-chloride groundwater beneath the northern Chihuahua desert, Trans-Pecos, Texas, USA. Hydrogeol J 5:4–16. https://doi.org/10.1007/s100400050102
Frape SK, Fritz P, McNutt RH (1984) Water-rock interaction and chemistry of groundwater from the Canadian Shield. Geochim Cosmochim Acta 48:1617–1627. https://doi.org/10.1016/0016-7037(84)90331-4
Frappart F, Hiernaux P, Guichard F, Mougin E, Kergoat L, Arjounin M, Lavenu F, Koité M, Paturel JE, Lebel T (2009) Rainfall regime across the Sahel band in the Gourma region, Mali. J Hydrol 375:128–142. https://doi.org/10.1016/j.jhydrol.2009.03.007
Freeze RA, Cherry JA (1979) Groundwater prentice. Englewood Cliffs, Englewood Cliffs
Fritz P (1997) Saline groundwater and brines in crystalline rocks: the contributions of John Andrews and Jean-Charles Fontes to the solution of a hydrogeological and geochemical problem. Appl Geochem 12:851–856. https://doi.org/10.1016/S0883-2927(97)00074-7
Gaillardet J, Dupré B, Louvat P, Allègre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159(1–4):3–30. https://doi.org/10.1016/S0009-2541(99)00031-5
Gombert P (1998) Synthèse sur la géologie et l’hydrogéologie de la série sédimentaire du sud est du Burkina Faso. Rapport technique, Programme RESO, ATG, IWACO, Ouagadougou
Groen J, Schuchmann JB, Geirnaert W (1988) The occurrence of high nitrate concentration in groundwater in villages in northwestern Burkina Faso. J Afr Earth Sci 7:999–1009. https://doi.org/10.1016/0899-5362(88)90013-9
Han G, Liu C (2004) Water geochemistry controlled by carbonate dissolution: a study of the river waters draining karst-dominated terrain, Guizhou Province, China. Chem Geol 204:1–21. https://doi.org/10.1016/j.chemgeo.2003.09.009
Huneau F, Dakouré D, Celle-Jeanton H, Vitvar J, Ito M, Traoré S, Compaoré NF, Jirakova H, Le Coustumer P (2011) Flow pattern and residence time of groundwater within the south-eastern Taoudeni sedimentary basin (Burkina Faso, Mali). J Hydrol 409:423–439. https://doi.org/10.1016/j.jhydrol.2011.08.043
Jeannin P-V, Hessenauer M, Malard A, Chapuis V (2016) Impact of global change on karst groundwater mineralization in the Jura Mountains. Sci Total Environ 541:1208–1221. https://doi.org/10.1016/j.scitotenv.2015.10.008
Kaiser HF (1960) The application of electronic computers to factor analysis. Educ Psychol Meas 20:141–151
Kessler JJ, Greerling C (1994) Profil environnemental du Burkina Faso. Université Agronomique, Département de l’Aménagement de la Nature. Wageningen, les Pays Bas, pp 63
Kirby CS, Cravotta CA III (2005) Net alkalinity and net acidity 1: theoretical considerations. Appl Geochem 20:1920–1940
Kortatsi BK, Tay CK, Anornu G, Hayford E, Dartey GA (2008) Hydrogeochemical evaluation of groundwater in the lower Offin basin, Ghana. Environ Geol 53(8):1651–1662. https://doi.org/10.1007/s00254-007-0772-0
Lavitt N, Acworth RI, Jankowski J (1977) Vertical hydrogeochemical zonation in a coastal section of the Botany Sands aquifer, Sydney, Australia. Hydrogeol J 5:4–74. https://doi.org/10.1007/s100400050117
Lee JY, Choi JC, Yi MJ, Kim JW, Cheon JY, Choi YK, Choi MJ, Lee KK (2005) Potential groundwater contamination with toxic metals in and around an abandoned Zn mine, Korea. Water Air Soil Pollut 165:167–185. https://doi.org/10.1007/s11270-005-4637-4
Li P, Zhang Y, Yang N, Jing L, Yu P (2016) Major ion chemistry and quality assessment of groundwater in and around a mountainous tourist town of China. Expo Health 8(2):239–252. https://doi.org/10.1007/s12403-016-0198-6
Li P, Tian R, Xue C, Wu J (2017) Progress, opportunities and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ Sci Pollut Res 24(15):13224–13234. https://doi.org/10.1007/s11356-017-8753-7
Marghade D, Malpe DB, Zade AB (2012) Major ion chemistry of shallow groundwater of a fast growing city of Central India. Environ Monit Assess 184:2405–2418. https://doi.org/10.1007/s10661-011-2126-3
Mazor E, Drever JI, Finley J, Huntoon PW, Lundy DA (1993) Hydrochemical implications of groundwater mixing: an example from the southern Laramie basin, Wyoming. Water Res Res 29:193–205. https://doi.org/10.1029/92WR01680
MEE (Ministry of Water and the Environment) (2001) Etat des lieux des ressources en eau au Burkina Faso et de leur cadre de gestion. Ouagadougou
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428. https://doi.org/10.2475/ajs.287.5.401
Morgenstern U, Daughney CJ (2012) Groundwater age for identification of baseline groundwater quality and impacts of land-use intensification—The National Groundwater Monitoring Programme of New Zealand. J Hydrol 456–457:79–93. https://doi.org/10.1016/j.jhydrol.2012.06.010
Ouédraogo C (1998) Cartographie géologique de la region Sud-Ouest du Burkina Faso au 1/200000—synthèse géologique. AQUATER/BUMIGEB
Pandey SK, Singh AK, Hasnain SI (2001) Hydrochemical characteristics of meltwater draining from Pindari glacier, Kumon Himalaya. J Geol Soc India 57:519–527
Patterson RA (1997) “Domestic Wastewater and the Sodium Factor”, site characterization and design of on-site septic systems. In: ASTM STP 1324, Bedinger MS, Johnson AI, Fleming JS (eds) American Society for Testing and Materials, 1997, pp 23–35
Piper AM (1944) A graphical procedure in the chemical interpretation of groundwater analysis. Trans Am Geo Union 25:914–928. https://doi.org/10.1029/TR025i006p00914
Prasanna MV, Chidambaram S, Shahul Hameed A, Srinivasamoorthy K (2011) Hydrogeochemical analysis and evaluation of groundwater quality in the Gadilam river basin, Tamil Nadu, India. J Earth Syst Sci 120:85–98. https://doi.org/10.1007/s12040-011-0004-6
Rail CD (2000) Groundwater contamination: sources and hydrology. CRC Press
Sarin MM, Krishnaswamy S, Dilli K, Somayajulu BLK, Moore WS (1989) Major ion chemistry of the Ganga-Brahmaputra river system: weathering processes and fluxes to the Bay of Bengal. Geochim Cosmochim Acta 53:997–1009. https://doi.org/10.1016/0016-7037(89)90205-6
Schoeller H (1962) Les Eaux Souterraines. Mason et Cie, Paris, p 642
Schoeller H (1965) Qualitative evaluation of groundwater resources. In: Methods and techniques of groundwater investigation and development. Water Resources Series No. 33, UNESCO, pp 44–52
Schoeller H (1967) Qualitative evaluation of ground water resources. In: Schoeller H (ed) Methods and techniques of groundwater investigation and development, Water Resource Series No. 33, UNESCO, Paris, pp 44–52
Schroeder HA (1960) Relations between hardness of water and death rates from certain chronic and degenerative diseases in the United States. J Chronic Diseases 12:586–591. https://doi.org/10.1016/0021-9681(60)90002-3
Singh AK, Hasnain SI (2002) Aspects of weathering and solute acquisition processes controlling chemistry of sub-alpine proglacial streams of Garhwal Himalaya, India. Hydrol Process 16:835–849. https://doi.org/10.1002/hyp.367
Smedley PL, Knudsen J, Maiga D (2007) Arsenic in groundwater from mineralized Proterozoic basement rocks of Burkina Faso. Appl Geochem 22:1074–1092. https://doi.org/10.1016/j.apgeochem.2007.01.001
Srinivasa Rao Y, Jugran DK (2003) Delineation of groundwater potential zones and zones of groundwater quality suitable for domestic purposes using remote sensing and GIS. Hydrol Sci J 48(5):821–833. https://doi.org/10.1623/hysj.48.5.821.51452
Stober I, Bucher K (1999) Deep groundwater in the crystalline basement of the Black Forest region. Appl Geochem 14:237–254. https://doi.org/10.1016/S0883-2927(98)00045-6
Subba RN, John Devadas D, Srinivasa Rao KV (2006) Interpretation of groundwater quality using principal component analysis from Anantapur district, Andhra Pradesh, India. Environ Geosci 13(4):239–259. https://doi.org/10.1306/eg.02090504043
Talbaoui M (2009) Etude des périmètres de protection des sources de Nasso et des forages ONEAI et ONEAII. Rapport de la mission de mars 2009, Programme de valorisation des ressources en eau de l’ouest VREO, p 24
Weaver TR, Frape SK, Cherry JA (1995) Recent cross-formational fluid flow and mixing in the shallow Michigan Basin. Geol Soci Am Bull 107:697–707. https://doi.org/10.1130/0016-7606(1995)107<0697:RCFFFA>2.3.CO;2
Wen X, Wu Y, Zhang Y, Liu F (2005) Hydro-chemical characteristics and salinity of groundwater in the Ejina Basin, Northwestern China. Environ Geol 48:665–675. https://doi.org/10.1007/s00254-005-0001-7
WHO (1999) Determination of hardness of water. Method WHO/M/26, RI
WHO (2006) Guidelines for drinking water quality, 3rd edn. World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland, pp 488–449
Wu J, Li P, Qian H, Duan Z, Zhang X (2014) Using correlation and multivariate statistical analysis to identify hydrogeochemical processes affecting the major ion chemistry of waters: case study in Laoheba phosphorite mine in Sichuan, China. Arab J Geosci 7(10):3973–3982. https://doi.org/10.1007/s12517-013-1057-4
Wu J, Wang L, Wang S, Tian R, Xue C, Feng W, Li Y (2017) Spatiotemporal variation of groundwater quality in an arid area experiencing long-term paper wastewater irrigation, northwest China. Environ Earth Sci 76(13):460. https://doi.org/10.1007/s12665-017-6787-2
Yameogo S, Savadogo AN (2002) Les Ouvrages de Captage de la ville de Ouagadougou Et Leur Vulnerabilite a la Pollution. In: Maiga AH, Pereira LS, Musy A (eds) Sustainable water resources management: health and productivity in hot climates. 5th inter-regional conference on environment and water
Yidana SM, Yidana A (2010) Assessing ground- water quality using water quality index and multivariate statistical analysis—the Voltaian basin, Ghana. J Environ Earth Sci 59:1461–1473. https://doi.org/10.1007/s12665-009-0132-3
Acknowledgements
We thank the Director general and laboratory staff of the Office National de l’Eau et de l’Assainissement (ONEA) in Ouagadougou for major cation and anion concentration analyses of the groundwater. We would like to thank Dr. Saga S. Sawadogo for producing Geological and groundwater sampling maps. Comments and suggestions from two anonymous reviewers greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sako, A., Yaro, J.M. & Bamba, O. Impacts of hydrogeochemical processes and anthropogenic activities on groundwater quality in the Upper Precambrian sedimentary aquifer of northwestern Burkina Faso. Appl Water Sci 8, 88 (2018). https://doi.org/10.1007/s13201-018-0735-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0735-5