Abstract
Since unsafe water is responsible for many illness, deaths, and economic failure, water quality monitoring is essential. A cross-sectional study was conducted on 218 drinking waters samples collected between February and June 2016 to assess water quality using phages by the help of CB390 E. coli host, plaque assay; multiple tube fermentation for coliforms and pour plate for heterotrophic bacteria at Ethiopian Public Health Institute. Heterotrophic plate count greater than 100 cfu/ml was noted in 41 samples and detections of total and thermotolerant coliforms and E. coli in 38, 24, and 10 samples, respectively, and no phages detection in chlorinated waters. While heterotrophic plate count greater than 100 cfu/ml was observed in 100 samples and detections of total and thermotolerant coliforms, E. coli, and phages in 75, 60, 42, and 5 samples, respectively, for untreated waters. The majority of the waters contained indicators above standard limits. This indicates that the sources are contaminated and they are potential threats for health. Hence, regular water monitoring should be a priority agenda.
Similar content being viewed by others
Introduction
Lack of safe water results in untold suffering, diseases, infant mortality, stunted growth, and economic loss (Pathak 2015). In developing countries, waterborne pathogens contribute 80% of health problems (Halvorson et al. 2010).
Since there is no universal indicator which has been identified (Skraber et al. 2004), testing of water using coliforms and coliphages would give a more complete picture of water quality. Due to cost constraints and the complexity of earlier coliphages methods, requiring testing for both indicators was previously not considered economically feasible (Salter 2012). Coliforms measure levels of fecal contamination in water distribution systems. They are facultative anaerobic, rod-shaped, Gram-negative bacteria, lactose fermenters, and produce acid and gas within 48 h at 35–37 °C (Rompre et al. 2002). Since coliforms respond to environment and water treatments, much less similar, and they have larger size compared to viruses (Nkwe et al. 2015), they are not representative of viral contamination (Jurzik et al. 2010). Total coliforms are mainly used for the assessment of sanitary water quality after the water has been treated and disinfected. Thermotolerant coliforms are more closely related to fecal pollution. Escherichia coli is the most specific to fecal contamination (Odonkor and Ampofo 2013).
Another fecal contamination monitoring tools in drinking water are coliphages. Of two main groups of coliphages, male-specific coliphages are either DNA or RNA viruses that infect E. coli via pilli. Somatic coliphages are DNA viruses that infect bacterial hosts by direct attachment to cell walls (Dryden et al. 2006). Single-agar layer CB390 method reduces cost and workload to detect and quantify total coliphages in water (Price 2014).
A secondary indicator employed to evaluate water quality is heterotrophic plate count (HPC). Heterotrophic bacteria are aerobic and facultative anaerobic organisms. The large number of the bacteria may suggest the presence of opportunistic pathogens of non-fecal origin, which possibly indicate taste, odour, and corrosion problems in the water distribution system (Islam and Dey 2014). In Ethiopia, water quality is assessed using bacterial indicators. However, the use of coliphage has not been assessed; hence, we set and objective to determine the quality of drinking water using total coliphages, E. coli, thermotolerant and total coliforms, and heterotrophic bacteria in selected water samples in Ethiopia.
Methods and materials
A cross-sectional study was conducted on 218 drinking water samples at Public Health Microbiology Research Team Laboratory in Ethiopian Public Health Institute between February and June 2016. Water samples were collected from 92 treated and 31 untreated piped, 69 untreated wells, 13 untreated rivers, and 13 bottled drinking water samples from different locations of Addis Ababa, Oromia, Amhara, Afar and Southern Nations, Nationalities, and Peoples’ Region (SNNPR) regional states of Ethiopia by trained professionals.
The study areas for pipes, wells, and rivers are located in central, northern, and southern Ethiopia that extend between latitude and longitude 8°00N and 38°00E, and present a diverse topography, ranging from 110 m below sea level to 4550 m above sea level.
The samples were collected into sterilized 500 ml bottles and transported on ice to the laboratory. The Samples containing residual chlorine were neutralized by adding pre-sterilized 0.5 ml sodium thiosulphate per 500 ml of water sample.
Carbon dioxide and filtration were the major treatment methods employed to inactivate or remove microbes in the carbonated bottled water industry and ultraviolet, ozone, and filtration for the non-carbonated water. Sampling site selection criteria were based on possible sources of contamination. Five units of the same batch of production of carbonated and uncarbonated bottled waters of eight brands produced in Ethiopia were collected to be analyzed as a single sample. Each bottle was thoroughly mixed to have an even distribution of microbes, and then, equal volume of the sample from each bottle of water was taken and mixed together to have a composite sample of the desired volume.
The samples were stored at 4 °C. Microbiological investigations were performed within 24 h after collection. All samples were tested for HPC, total and thermotolerant coliforms, E. coli, male-specific, and somatic coliphages using standard methods (Price 2014; HACH 2012; Cheesbrough 2006); US Environmental Protection Agency 2001).
Hpc assay
One millilitre of each water sample was pipetted into the sterile petri dish. After thoroughly mixing, the melted plate count agar was poured into the dish. The melted medium was mixed thoroughly with the sample and let them solidify. The plates were incubated for 48 h at 35 °C (HACH 2012).
Coliform assay
The total coliform count test was based on the multiple tube fermentation method to estimate the most probable number (MPN) of total coliform, thermotolerant coliform and E. coli in 100 ml of water. The test was carried out by incubating at 37 °C for 48 h in measured quantities of sample water (50, 10, and 1 ml) into tubes of double and single-strength MacConkey broth (Difco). The tubes with acid and gas production were inoculated in brilliant green lactose bile broth to observe gas production after 48 h of incubation at 37 °C. Then, E. coli and nutrient broths were inoculated and incubated at 44.5 °C for 48 h for gas production and indole test, respectively. The coliforms were estimated per 100 ml of water using MPN tables (Cheesbrough 2006).
Coliphages assay
For the simultaneous detection of both types of male-specific and somatic Coliphages, single-agar layer plaque assay using coliphage host CB390 (obtained from University of North Carolina, Chapel Hill) log phase containing 0.15% ampicillin was applied with magnesium chloride in double-strength tryptic soy agar [Difco]. The coliphages were incubated for 16–24 h at 37 °C and the plaques were enumerated. The coliphage counted was computed per 100 ml of the sample (Price 2014; US Environmental Protection Agency 2001).
Data analysis procedures
The data were entered, cleaned, and analyzed using SPSS 20 for Windows (SPSS Inc. Version 20, Chicago, Illinois). The Kruskall–Wallis test was used to find out the differences in indicators values by regions, water sources, and treatment types; and to observe the associations among the various indicators; the Spearman Rank Correlation was used to test the association between the variables. To check the normality of data, Shapiro–Wilk test was used. The significance level was set at p value < 0.05. The results were compared with established Ethiopian standards and WHO guidelines of drinking water.
Results
Microbial quality of water samples
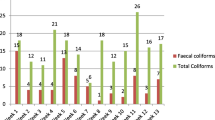
Of the total 218 samples tested from various waters, heterotrophic bacteria, total and thermotolerant coliforms, E. coli, and total coliphages were detected in 72.9% (n = 159), 51.8% (n = 113), 38.5% (n = 84), 23.9% (n = 52), and 2.3% (n = 5) of the samples, respectively. HPC > 100 cfu/ml were observed in 141 (64.7%) samples. High counts (> 3.0 × 102 cfu/ml) were found in 22.5% (n = 49) of all samples for HPC; > 180 MPN/100 ml in 17% (n = 37) of the samples for total coliform and 11.5% (n = 25) of the samples for thermotolerant coliforms.
Of the total water samples for chlorinated water samples, HPC > 100 Cfu/ml were observed in 41 (18.8%) samples; detections of total and thermotolerant coliforms and E. coli in 38 (17.4%), 24 (11.0%), and 10 (4.6%) samples, respectively, and no detection of coliphages. While, for untreated waters, HPC > 100 cfu/ml were observed in 100 (45.9%) samples; detections of total and thermotolerant coliforms, E. coli, and coliphages in 75 (34.4%), 60 (27.5%), 42 (19.3%), and 5 (2.3%) samples, respectively. In all coliphage-detected samples, all indicators, heterotrophic bacteria, and coliforms were also detected, inversely in the absence of thermotolerant coliforms, there were no coliphages detections. The detections of heterotrophic bacteria, total coliforms, and thermotolerant coliforms by treatment status of water samples were shown in Tables 1, 2, and 3.
Rho values of HPC, and total and thermotolerant coliforms to coliphages were 0.202, 0.232, and 0.269; total and thermotolerant coliforms to HPC were 0.754 and 0.677, respectively, and total coliforms to thermotolerant was 0.816. p values for HPC, and total and thermotolerant coliforms and coliphages using Kruskall–Wallis test for the samples by region, source, and treatment type were < 0.05.
Bacteriological and virological indicator detection in treated water sources
Of chlorinated drinking waters, 41(44.6%) samples had HPC > 100 cfu/ml and 38 (41.3%), 24 (26.1%), and 10 (10.9%) samples were positive for total and thermotolerant coliforms and E. coli, respectively, and no detection of coliphages. All eight brands of 13 bottled drinking water samples in Addis Ababa (N = 4) and Oromia (N = 9) had HPC < 100 cfu/ml; total and thermotolerant coliforms and E. coli of < 1 MPN/100 ml and coliphages of < 1 pfu/100 ml.
Bacteriological and virological indicator in untreated waters
Of untreated drinking waters, 100 (88.5%) samples had HPC > 100 cfu/ml and 75 (66.4%), 60 (53.1%), 42 (37.2%), and 5 (4.4%) samples were positive for total and thermotolerant coliforms, E. coli, and coliphages, respectively. Untreated waters had HPC ranging from 100% for river to 80.6% for untreated piped water, total coliforms ranging from 92.3% for rivers to 60.9% for wells, thermotolerant coliforms ranging from 92.3% for rivers to 45.1% for piped waters, E. coli ranging from 84.6% for rivers to 29% for wells, and coliphages ranging from 38.5% for rivers to no detection for piped and well waters.
Of unchlorinated piped waters, 25 (80.6%) samples had HPC > 100 cfu/ml; 21 (67.7%), 14 (45.1%), and 11 (35.5%) samples were positive for total and thermotolerant coliforms and E. coli, respectively and no detection of coliphages in the samples.
Of unchlorinated wells, 62 (89.9%) samples had HPC > 100 cfu/ml; 42 (60.9%) samples, 34 (49.3%) samples, and 20 (29%) samples were positive for total and thermotolerant coliforms and E. coli, respectively and no detection of coliphages in the samples.
Of the rivers, all samples had HPC > 100 cfu/ml and 12 (92.3%), 12 (92.3%), 11 (84.6%), and 5 (38.5%) samples were positive for total and thermotolerant coliforms, E. coli, and coliphages respectively. The enumeration of coliphages analyzed in water samples from rivers were ranged between 2.2 × 101 and 5.3 × 102 pfu/100.
Discussion
In the present investigation, virological and bacteriological quality of drinking water was assessed from various sources in Ethiopia. As expected, the majority of the samples (64.7%) contained HPC above permissible limit of > 100 cfu/ml (WHO 2003) and total coliforms (51.8%), thermotolerant coliforms (38.5%), (23.9%), and coliphages (2.3%) above WHO guideline value of < 1/100 ml (WHO 2011) of the total water samples. The presence of these indicators may not cause illness, but used as one of the indicators of pathogens that can cause intestinal infections, hepatitis, typhoid fever, and other illnesses (Emmanuel et al. 2009).
The findings observed in the present study about the incidences above the permissible limits of HPC and total coliforms in the total samples were lower than in some studies done on drinking water samples in Peshawar, Pakistan (80 and 70%) (Ali et al. 2013) but higher than in one study in Pakistan (45 and 25%) (Shar et al. 2010).
The presence of total coliforms in the chlorinated drinking water samples indicates a serious treatment failure; distribution system may be vulnerable to contamination (Ainsworth 2004; Health Canada 2010). Out of water samples positive for thermotolerant coliforms in the present study, 28.6% were treated drinking waters which indicate inadequate treatment and disinfection (Osman et al. 2011). The presence of E. coli in samples was more serious than other coliforms alone and possible presence of pathogens (Odonkor and Ampofo 2013).
The non-comply of 38.5% drinking water samples for thermotolerant coliforms is higher than the national survey, rapid assessment of drinking water quality in Ethiopia that tested drinking water quality for thermotolerant coliforms and found 28% of 1602 samples non-potable (Tadesse et al. 2010).
The finding observed in the present study about the presence of thermotolerant coliforms or E. coli above the recommended limits was comparable to similar study conducted in Bangladesh in various waters (Parvez et al. 2016) and lower than the study in India (100% and 78.1% (Borah et al. 2010). Though, in one study, the detections of these bacterial indicators were higher than that from Lithuania (16.7%) (Malakauskas et al. 2007).
The detections above permissible limits for HPC, total and thermotolerant coliforms, E. coli, and coliphages in untreated water samples were 2.7 times, 2.0 times, 2.5 times, 4.2 times, and 4.4 times more than the detections for chlorinated water samples, respectively. The non-detection of coliphages in any treated water samples is consistent with the study done in the United States on ground water (Plummer et al. 2014).
Spearman correlation test indicated statistically significant positive correlations between coliphages and all other bacterial indicators and agreed with a study done in the United States on drinking water samples (Wu et al. 2011). HPC, and total and thermotolerant coliforms were strongly related to each other, though the non-parametric Kruskall–Wallis test showed that HPC, coliforms and total coliphages differed statistically by region, water source, and treatment type (p value < 0.05).
In the present study, the non-compliance of samples collected from chlorinated piped water for thermotolerant coliforms was high (26.1%) compared to 22.4% in survey of the same country. This might be due to the increment of sanitary risk factors such as lack of maintenance, leaks in piped water distribution system; poor site selection and failure to minimize sanitary risks (Tadesse et al. 2010).
All the 13 samples from the eight brands of bottled water contained < 100 cfu/ml for HPC and no detectable organism per 100 ml for coliforms and coliphages. This good quality may be due to the current follow-up and enforcement of the bottled water companies to adhere to good manufacturing practice by the regulatory body. This is in consistent with the study done in Ghana (Addo et al. 2009) which found coliforms none detectable in any of the examined brands of bottled drinking waters.
Some investigations done in different countries have contained indicators in bottled drinking water. In Ethiopia, 325 bottled water samples of 11 domestic and two imported brands contained HPC above the permissible limits in 16.9% of the samples (Tafere et al. 2014). In India, Jaipur city, 40 samples of various brands of bottled drinking water were tested for HPC, total coliforms and E. coli reported 50, 45, and 20%, respectively (Gangil et al. 2013). In study done in Dar es Salaam, on 13 brands of 130 bottled drinking water, 92% was reported HPC, 4.6% total coliforms, and 3.6% thermotolerant coliforms (Kassenga 2007).
As expected, HPC non-compliance for treated piped water was lower than untreated water sources. Non-compliance of thermotolerant coliforms for treated piped water in Addis Ababa was lower than non-compliance in Oromia piped water but consistent with the previous national survey of RADWQ (Tadesse et al. 2010).
Large non-comply of untreated water sources for HPC (88.5%), total coliforms (66.4%), thermotolerant coliforms (53.1%), E. coli (37.2%), and total coliphages (4.4%) signified the suggestion of WHO which states that surface water or shallow ground water should not be used as a source of drinking water without sanitary protection or treatment (WHO 2011).
The contamination of HPC and coliforms in untreated piped water was higher than that of treated piped water. The presence of E. coli in untreated piped water (35.5%) was 3.3 times higher than in treated piped water samples. The detections of total and thermotolerant coliforms and E. coli in 60.9, 49.3, and 29%, respectively, in wells were lower than studies done in Saudi Arabia (100% total coliform, 87.88% thermotolerant coliforms) (Al Otaibi 2009) and Nigeria (100% total coliform and 65% E. coli) (Nwachukwu and Ume 2013). On the other hand, in some studies, the detections of total and thermotolerant coliforms and E. coli were higher than that from Turkey (25% total coliform, 17.5% thermotolerant coliforms and 15% E. coli (Aydin 2007).
Thermotolerant coliforms in protected dug wells (49.3%) were higher than untreated and treated piped waters and comparable with the previous study in Ethiopia (Tadesse et al. 2010). Non-compliance of thermotolerant coliforms in Addis Ababa protected dug wells (33.3%) and Oromia protected dug wells (54.9%) was higher than the previous study in Ethiopia (25%) for both regions (Tadesse et al. 2010). This level of contamination may be due to a source of contamination which is nearby; otherwise, groundwater sources are usually bacteriologically safe (Nkwe et al. 2015).
The non-compliance of HPC, total and thermotolerant coliforms, E. coli, and total coliphages in 100, 92.3, 92.3, 84.6, and 38.5%, respectively, in rivers indicates that they are the most contaminated sources and unfit for consumption. This poor quality of the source was consistent with the study done in Kathmandu Valley, Nepal (Haramoto et al. 2011). Rivers samples in SNNPR were more contaminated with the indicators than river samples in Oromia in the present study. The presences of coliphages in the rivers indicate the presence of enteric viruses (Plummer et al. 2014).
Conclusion
This study assessed bacterial and viral quality of drinking water sources in some regions of Ethiopia. Majority of the waters, mainly untreated sources contained bacterial indicators and rivers contained viral indicators above the standards permissible limits. This indicated that drinking waters are contaminated with environmental and fecal contaminants which are potential threats for human health, and probably, some of the waters sources may be unsafe for human consumption. This shows the high risk of infection for consumers and calls for immediate action. We recommend regular microbiological assessment and strengthening the water treatment system will reduce the risk of communicable diseases.
References
Addo KK, Mensah GI, Donkor B, Bonsu C, Akyeh ML (2009) Bacteriological quality of bottled water sold on the Ghanaian market. AJFAND 9:1378–1387
Ainsworth R (2004) Safe piped water: managing microbial water quality in piped distribution systems. IWA Publishing, London, UK
Ali J, Ullah N, Ali KF, Rahman Z, Ahmad I, Hassan S, Ahmad I (2013) Bacteriological quality analysis of drinking water of rural areas of Peshawar, Pakistan. Am Eur J Agric Environ Sci 13(9):1202–1206
AlOtaibi ELS (2009) Bacteriological assessment of urban water sources in Khamis Mushait Governorate, southwestern Saudi Arabia. Int J Health Geogr. https://doi.org/10.1186/1476-072X-8-16
Aydin A (2007) The microbiological and physico-chemical quality of groundwater in West Thrace, Turkey. Pol J Environ Stud 16:377–383
Borah M, Dutta J, Kumar Abani, Misra AK (2010) The bacteriological quality of drinking water in Golaghat Sub-division of Golaghat District, Assam, India. Int J Chemtech Res 2:1843–1851
Cheesbrough M (2006) District laboratory practice in tropical countries, 2nd edn. Cambridge University Press, Cambridge
Dryden SK, Ramaswami B, Yuan Z, Giammar DE, Dryden SK, Angenent LT (2006) A rapid reverse transcription-PCR assay for F + RNA coliphages to trace fecal pollution in Table Rock Lake on the Arkansas–Missouri Border. Water Res 40:3719–3724
Emmanuel E, Pierre MG, Perrodin Y (2009) Groundwater contamination by microbiological and chemical substances released from hospital wastewater and health risk assessment for drinking water consumers. Environ Int J 35:718–726
Gangil R, Tripathi R, Patyal A, Dutta P, Mathur KN (2013) Bacteriological evaluation of packaged bottled water sold at Jaipur city and its public health significance. Vet World 6:27–30
HACH (2012) Standard Methods for the examination of water and wastewater: method 9215 B, Pour Plate Method. HACH, USA
Halvorson SJ, Williams AL, Ba S, Dunkel FV (2010) Water quality and water borne disease in the Niger River Inland Delta, Mali: a study of local knowledge and response. Health Place 17:449–457. https://doi.org/10.1016/j.healthplace.2010.10.002
Haramoto E, Yamada K, Nishida K (2011) Prevalence of protozoa, viruses, coliphages and indicator bacteria in groundwater and river water in the Kathmandu Valley. Nepal RSTMH 105:711–716
Health Canada (2010) Canadian drinking water guidelines. Health Canada, Ottawa, ON. http://www.hc-sc.gc.ca/ewh-semt/water-eau/drink-potab/guide/index-eng.php. Accessed 10 Mar 2016
Islam MS, Dey SK (2014) Designing of culture media for increased recovery of total heterotrophs from water samples. IJIMS 1:147–153. http://www.ijims.com. Accessed 10 Mar 2016
Jurzik L, Hamza IA, Puchert W, Überla K, Wilhelm M (2010) Chemical and microbiological parameters as possible indicators for human enteric viruses in surface water. Int J Hyg Environ Health 213:210–216
Kassenga GR (2007) The health-related microbiological quality of bottled drinking water sold in Dar es Salaam, Tanzania. J Water Health 5:179–185
Malakauskas M, Kasnauskytė N, Kudirkienė E, Šernienė L, Malakauskas A, Stimbirys A, Milius J (2007) Microbiological evaluation of drinking water from centralized and small community supply systems in Kaunas region, Lithuania. Vet Med Zoot 38:50–56
Nkwe KI, Ateba CN, Sithebe NP, Bezuidenhout CC (2015) Enumeration of somatic and F-RNA phages as an indicator of fecal contamination in potable water from rural areas of the North West Province. Pathog 4:503–512
Nwachukwu E, Ume CA (2013) Bacteriological and physicochemical qualities of drinking water sources in local area of Eastern Nigeria. J Soc Social Work Res 2:336–341
Odonkor ST, Ampofo JK (2013) Escherichia coli as an indicator of bacteriological quality of water—an overview. Microbiol Res 4:5–11
Osman GA, Kamel MM, Hassan HM, Al-Herrawy AZ (2011) Microbial quality of nile water and drinking water in some areas of Greater Cairo. Egypt Aust J Basic Appl Sci 5(11):1328–1334
Parvez AK, Liza SM, Marzan M, Alvee Ahmed A, Rahman MS (2016) Bacteriological quality of drinking water samples across Bangladesh. Arch Clin Microbiol 7:1
Pathak H (2015) Effect of water borne diseases on indian economy: a cost- benefit analysis. An Rom Sov Ser Med Gen 1:74–78
Plummer JD, Long SC, Liu Z, Charest AA (2014) Torque Teno virus occurrence and relationship to bacterial and viral indicators in feces, wastewaters, and waters in the United States. J Environ Health Sci 31:671–680
Price MT (2014) Comparison of Single-Agar Layer and two-step enrichment spot plate methods in the detection of somatic and male-specific coliphages in NC type II reclaimed water samples. Master’s thesis, University of North Carolina, Chapel Hill, NC
Rompre A, Servais P, Baudart J, De-roubin MR, Laurent P (2002) Detection and enumeration of coliforms in drinking water: current methods and emerging approaches. J Microbiol Methods 49:31–54
Salter R (2012) Testing drinking water for coliphage as a fecal quality indicator: advances in rapid coliphage detection. Charm Sciences, Westbrook
Shar AH, Kazi YF, Kanhar NA, Soomro IH, Zia SM, Ghumro PB (2010) Drinking water quality in Rohri City, Sindh, Pakistan. Afr J Biotechnol 9:7102–7107
Skraber S, Gassilloud B, Gantzer C (2004) Comparison of coliforms and coliphages as tools for assessment of viral contamination in river water. Appl Environ Microbiol 70:3644–3649
Tadesse D, Desta A, Geyid A, Girma W, Fisseha S, Schmoll O (2010) Rapid assessment of drinking water quality in the federal democratic republic of Ethiopia. WHO and UNICEF, Geneva, Switzerland
Tafere W, Abera F, Beyene Y, Legesse T (2014) Microbiological quality and safety of bottled water brands sold in Ethiopia. Ethiop J Health Dev 28:178–184
US Environmental Protection Agency (2001) Method 1602: male-specific (F_) and somatic coliphage in water by single agar layer (SAL) procedure. EPA document 821-R-01-029. Environmental Protection Agency, Washington
WHO (2003) Heterotrophic plate counts and drinking-water safety: the significance of HPCS for water quality and human health. WHO Health Organization, UK
WHO (2011) Guidelines for drinking water quality, recommendations, 4th edn. World Health Organization, Switzerland
Wu J, Long SC, Das D, Dorner SM (2011) Are microbial indicators and pathogens correlated? A statistical analysis of 40 years of research. J Water Health 9:265–278
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bedada, T.L., Mezemir, W.D., Dera, F.A. et al. Virological and bacteriological quality of drinking water in Ethiopia. Appl Water Sci 8, 70 (2018). https://doi.org/10.1007/s13201-018-0716-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0716-8