Abstract
Surface waters, especially rivers are the most important sources of water supply for drinking and agricultural purposes. Water with desirable quality is necessary for human life. Therefore, knowledge of water quality and its temporal changes is of particular importance in sustainable management of water resources. In this study, available data during 20 years from two hydrometry stations located in the way of Horroud River in Lorestan province were used and analyzed using Aq.QA software. Piper, Schoeller, Stiff, and Wilcox diagram were drawn and Mann–Kendal test was used for determining data trend. According to Wilcox diagram, water of this river in both stations is placed in c2s1 class which is good for agricultural purposes, and according to Schoeller diagram, there is no restrict for drinking purposes. Results of Man–Kendal test show increasing trend for colorine, EC, TDS while decreasing trend for potassium in Kakareza station. On the other hand in Dehnu station, positive trend was seen in calcium and colorine while negative trend for sulfate and potassium. For other variables, no specific trend was found.
Similar content being viewed by others
Introduction
Water quality is a growing concern throughout the developing world. Drinking water sources are under increasing threat from contamination, with far-reaching consequences for the health of children and for the economic and social development of communities and nations (UNICEF handbook of water quality, 2008). Quality of water is part of the ecological concerns to be considered in the beginning, and knowledge of irrigation water is critical to understand what management changes are necessary for long-term productivity (Bohn et al. 1985; Brady 2002 cited in Gebrehiwot et al. 2011). Rivers, as the main sources of drinking water in urban and rural regions, play an important role in human health and environment safety. However, by improper and irregular exploitation of stream water during recent years, man caused pollution in the most fundamental living liquid (Afshar 2006; Allahyari pour and Mohsenifar 2011). Many researchers, such as Pourmohammad and Rahimi Nejad in 1997, Zipper et al. 2002, Soltani 2006a, b, Ozcan 2007, Sadashivaiah 2008, Allahyaripour and Mohsenifar 2011 conducted their surveys on water quality in different parts of the world. In their study, Pourmohammad and Rahimi Nejad (1997) investigated changes of water quality in Zayandehrood and concluded that the salinity of this water in the lowermost part of the river has been increased dramatically so that it is not suitable for being used in agriculture. Soltani (2006a, b) studied the water quality of Zayandehrood for agricultural purposes, and in all stations, from Ahvaz to Khorramshahr, the water quality according to exchangeable sodium ion was grouped in class 1 and its salinity in class 3. Studying the quality of ground waters of Tumkur, Karnataka, India, Sadashivaiah (2008) drew Piper diagram for two stations and determined the type of water for studied area for Ca–Mg–HCO3.
Allahyaripour and Mohsenifar (2011) conducted a survey on Quality Determination of Zayandehrood river with Aq.qa and results indicated that the water of initial stations (near the origin of spring) was of Ca–HCO3 type with average degree of salinity. Qishlaqi et al. 2016, in their study, assessed the physicochemical characteristics of the Tireh River water in Lorestan, Iran, and demonstrated that almost all samples have suitable conditions for drinking with regard to the WHO standard and in comparison with agricultural standard (FAO Standard), and the potential of water is suitable for irrigation purposes.
The assessment of long-term water quality changes is also a challenging problem. During the last decades, there has been an increasing demand for monitoring water quality of many rivers by regular measurements of various water quality variables. The result has been the gradual accumulation of reliable long-term water quality records and the examination of these data for long-term trends (Hirsch et al. 1991). Long-term trend of water quality in natural systems reveals information about chemical and biological changes, and variations due to man-made and/or seasonal interventions (Hipel 1985). Various studies were also conducted on trend analysis. Hirsch et al. 1982, Miller and Hirst 1998, Antonopoulos et al. 2001, Boyacioglu and Boyacioglu 2008 and Kauffman and Belden 2010 worked on trend analysis of water quality chemical parameters. Routine water quality monitoring data from six stations in the Spokane River Basin by Washington state department of ecology (1979) were analyzed using a trend detection technique which employed data deseasonalization and non-parametric statistical analysis. Trend analysis reveals decreasing zinc concentration by approximately 50% between 1973 and 1978 and decreasing trend for phosphate from 1972 to 1975, followed by an increasing trend from about 1975 to late 1977. Analysis of phosphate loading at Riverside State Park reveals no long-term trends. The results of a survey in trend changes in Golroad river from Nekarood river in Mazandaran using recorded data for 13 variables during 35 years by Salarian et al. (2013a, b) showed that in most cases there were no trends for anions and cations but both anions and cation summation had a trend.
The aim of this study is determining water quality alongside Horroud River for agricultural and drinking purpose and detecting any probable trend in ion concentration.
Materials and methods
Study area
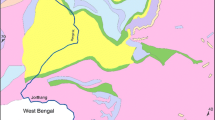
Horroud River with approximately 107 km length is a mountainous river located in the east part of Khorramabad city in Lorestan province. It is one of the main branches of Karkheh Basin and its surrounded area is covered with agricultural lands. Human structures, such as roads, farmlands, and rural settlements, are built on its terraces. Due to a conglomerate formation, most of its sediments are coarse (Yamani and Sharafi 2012). This river stands between latitude and longitude 32°22′ to 33°52′N and 48°15′ to 49°E, respectively. Position of the case study in Lorestan province in Iran is shown in Table 1 and Fig. 1.
Data collection
For this study, the information of two hydrometry stations (Kakareza and Dehnu) along Horroud River including cations (calcium, magnesium, sodium, and potassium) and anions (bicarbonates, chlorine, and sulfate) in terms of milli equivalent per liter (mEq/L), electrical conductivity, and pH within the years 1992–2011 were taken from Water Authorities of Khorramabad. Tables 2 and 3 below show some statistical parameters and correlation coefficient between ion concentrations.
Water quality
Piper and stiff diagram
Piper diagram is a combination of anions and cations triangle that lies on a common baseline. It divides waters into basic types according to their placement near the four corners of the diamond. Water that plots at the top of the diamond is high in Ca2++, Mg2+, and HCO3−, and is the region of waters with temporary hardness. Waters plotted at the lower corner is the diamond composed primarily of Na2+, K+, and HCO3− + CO3 (Fig. 2). Stiff diagrams show the composition of a single sample, in terms of common cation and anion, with concentration represented in electrical equivalents. These two diagrams can be used for fast determining water type.
Salinity hazard
This parameter is determined according to the following table (Table 4). The program uses measured conductivity, if available, or measured dissolved solids, or calculated dissolved solids.
Sodium adsorption ratio
The sodium adsorption ratio (SAR) is calculated according to Eq. 1.
where [] represents concentration in units of mEq/L.
Exchangeable sodium ratio
The exchangeable sodium ratio (ESR) is calculated according to Eq. 2.
where [] represents concentration in units of meq/L.
Magnesium hazard
The magnesium hazard (MH) is calculated as Eq. 3.
where [] represents concentration in units of meq/L
Hardness
Hardness is the sum of Ca2+ and Mg2+ concentrations expressed in terms of mg/l of calcium carbonate. Hardness can be calculated as Eq. 4.
The degree of hardness in water is commonly based on the classification listed in Table 5 (Sawyer and Mc Carty, 1967).
Trend
In this study for the examination and recognition of series temporal changes, T statistic of Kendal test and statistical–graphical method of Mann–Kendal was used, and time and type of data changes were determined.
Mann–Kendall test is a non-parametric test which is used to detect monotonic trends in hydrologic data analysis. Trends detectable by the Mann–Kendall method are not necessarily linear.
First, the test of the data being random was conducted using the Mann–Kendall method (recommended by the global meteorology department) to determine the existence or nonexistence of any sort of the data trend. To conduct this test, first statistical series are ranked and the following equation is used (Eq. 5):
For determining the variation or the trend, where T is the statistic of the Mann–Kendall, n is the total number of statistical questions and p is the sum of the number of ranks greater than each of the class of ni that is placed after that and is obtained from Eq. 6.
To measure the significance of the statistic, T is calculated from Eq. 7 which is mentioned below:
where the critical point for 20 years is 0.32.
If: \( - 0.32 < T < + 0.32 \), no important trend is seen in the series and they are random. Also, if \( T < \left( T \right)_{t} \) or \( T < - 0.32 \) means that the trend is negative in the series and if T \( > \left( T \right)_{t} \) or T \( > + 0.32 \), the trend in the series is positive.
To determine the direction of the trend, type, and the time of variation, we need a Kendall graphic test. First, data are ranked, and the statistic of \( t_{i}^{{\prime }} \) (I rank ratio to prior ranks) is calculated, then the cumulative frequency of the statistic of \( \left( {\sum t_{i} } \right)t_{i} \) is obtained. Later, the expected value, variance, and the Mann–Kendall index are calculated based on the formulas 8, 9, and 10, respectively.
The values of u are significant when the rising and declining trend is observed in it and this depends on whether its value is greater or smaller than zero, respectively (\( U_{i} > 0\; {\text{or}}\;\; U_{i} < 0) \). To investigate the variations, the index \( u_{i}^{{\prime }} \) should be determined. The process of calculating \( u_{i}^{{\prime }} \) is as follows: data are ranked and the statistic of \( t_{i}^{{\prime }} \) (I rank ratio to following ranks) is determined and then, the cumulative frequency of \( \left( {\sum t_{i} } \right)t_{i} \) is calculated. The expected value and the index of \( u_{i}^{{\prime }} \) are calculated as Eqs. 11, 12, and 13.
Results and discussion
Water quality determination
For analyzing water quality, Aq.QA software was used, and Piper, Stiff, Schoeller, and Wilcox diagram were drawn. Salinity, SAR, ESR, magnesium hazard, hardness, and correlation coefficient between ions were also detected.
According to stiff diagrams water type in both stations during these 20 years was Ca–HCO3 and hasn’t changed, except in 1997 in Dehnu station which turns to Mg–HCO3 (Fig. 3). In Table 6, the result of calculate value of salinity hazard, SAR, ESR, magnesium hazard, TH (mg/l), and also water type in each year within the studied period is mentioned. As it is illustrated, water in both stations placed in hard water classes with medium salinity.
To determine the viability of water for irrigation and drinking purposes, Wilcox and Schoeller diagram were also used, respectively. According to Wilcox plot in both stations, water was plotted in the C2S1 zone, indicating low sodium absorption ratio (SAR) and medium ECw; so according to result in both stations, water quality for irrigation is good. In estimating drinking purpose, Schoeller diagram, a semi-logarithmic diagram of the concentrations of the main ionic constituents in water (SO4, HCO3, Cl, Mg, Ca, and Na/K), was plotted and showed good quality for drinking purposes.
Trend detection
The varying time of temporal series is a place where from then on, another statistical distribution prevails over the data (Ha and Ha, 2006). The point of confluence of the two diagrams of u and u′ indicates a substantial point of variation and the existence of the trend. In other words, if the said lines intersect each other inside the critical range of (± 1.96) it is a sign of sudden change in the data and if the lines intersect outside of the critical range of (± 1.96) it is a sign of the existence of the trend in a temporal series (Sueyers, 1990). The behavior of i u after the point of confluence shows the trend situation (rising and declining). The lack of point of confluence of the two indexes is a sign of lack of variation in the temporal series (Turkes, et al., 2002). According to Man-Kendall analysis in Kakareza station increasing trend was illustrated for colorine, EC and TDS while decreasing trend for potassium. On the other hand in Dehnu station positive trend was seen in calcium and colorine, while negative trend for sulfat and potassium. For other variables no specific trend was found (Figs. 4 and 5).
Conclusion
Measured values of 11 quality variables (Ca, Mg, Na, K, HCO3,Cl, SO4,TDS, EC, and PH) at two hydrometry stations (Dehnu and Kakareza) of Horroud River in Lorestan province in Iran for a period of 20 years were analyzed using Aq.QA software and Mann–Kendal test. Result indicated that water of this river in both stations is good for agriculture and drinking purposes. In trend investigation, Mann–Kendal test shows increasing and decreasing trend for colorine and potassium, respectively, in both stations. Uptrending values for TDS and EC wére seen in Kakareza station. In Dehnu station, also positive gradient was seen for calcium and positive gradient for sulfate. In general, we can notice that although in some variables specific trend was seen, water type has not changed during these 20 years, except in 1997 in Dehnu station which turns to Mg-Hco3. Also in both stations, water was categorized in hard water class with medium salinity hazard.
References
Afshar M (2006) Karun river development cane, Tenth International Egyptian Water Technology Conference
Allahyaripour F, Mohsenifar K (2011) Quality determination of Zayandehroud river with Aq. qa in Iran. Adv Environ Biol 5(8):2469–2474
Antonopoulos V, Papamichail M, Mitsiou K (2001) Statistical and trend analysis of water quality and quantity data for the Strymon River in Greece. Hydrol Earth Syst Sci 5(4):679–691
Boyacioglu H, Boyacioglu H (2008) Investigation of temporal trends in hydro chemical quality of surface water in western Turkey. Bull Environ Contam Toxicol 80:469–474
Gebrehiwot A, Tadesse N, Bheemalingeswara K, Haileselassie M (2011) Suitability of groundwater quality for irrigation: a case study on hand dug wells in hantebet catchment, Tigray, Northern Ethiopia. J Am Sci 7(8)
Ha JK, Ha E (2006) Climatic change and inter annual fluctuation in the long-term record of monthly precipitation for Seoul. Int J Climatol 26:607–618
Hipel KW (1985) Time series analysis in perspective. Water Resour Bull 21:609–624
Hirsch RM, Slake JR, Smith RA (1982) Techniques of trend analysis foe monthly water quality data. Water Resour Res 18:107–121
Hirsch RM, Alexander RB, Smith RA (1991) Selection of methods for the detection and estimation of trends in water quality. Water Resour Res 27:803–813
Kauffman GJ, Belden AC (2010) Water quality trends (1970 to 2005) along Delaware streams in the Delaware and Chesapeake Bay watersheds, USA. Water Air Soil Pollut 208:345–375
Miller JD, Hirst D (1998) Trends in concentrations of solutes in an upland catchment in Scotland. Sci Total Environ 216:77–88
Ozcan H (2007) Assessment of the water quality of troia for the multipurpose usages. Springer Science. Environ Monit Assess 130:389–402
Qishlaqi A, Kordian S, Parsaie A (2016) Hydrochemical evaluation of river water quality—a case study. Appl Water Sci (Springer). doi:10.1007/s13201-016-0409-0
Sadashivaiah C (2008) Hydrochemical analysis and evaluation of groundwater quality in Tumkur Taluk, Karnataka, India. Environ. Res
Salarian M, Ansari H, Chaparli H (2013a) Analysis of trend changes in Golroad river from Nekaroad River in Mazandaran
Salarian M, Jamshidi A, Alizade A (2013b) Quality trend of Soleimantange, Kordkhil and Rigcheshme in Mazandaran using Mann–Kendall non-parametric test
Sawyer CN, Mc Carty PL (1967) Chemistry for sanitary engineers, and classification of naturally soft and naturally hard waters to sources and hardness of their water supplies. J Hyg
Soltani MA (2006a) Karun River Water Quality Assessment of Agricultural Ahaz range in Khorramshahr, Ahwaz
Soltani MA (2006b) Studying water quality of Zayandeh rood for agricultural purposes
Sueyers R (1990) On the statistical analysis of series of observation. WMO 415:2–15
Turkes M, Sumer UM, Demir I (2002) Re-evaluation of trends and changes in mean. maximum and minimum temperatures of Turkey for the Period 1929–1999. Int J Climatol 22:947–977
Unicef handbook on water quality, NewYork, 2008
Yamani M, Sharafi S (2012) Geomorphology and effective factor in riverbank erosion of Horrood River in Lorestan. Geography and Environmental Programming (33):1, 15–32
Zipper C, Holtzman E, Golde I, Patrick D, Gildea F, Stewart JJ, Roger E (2002) Virginia USA Water Quality, 1978 to 1995: regional Interpretation. J Am Water Resour Assoc 38(3):789–802
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Falah, F., Haghizadeh, A. Hydrochemical evaluation of river water quality—a case study: Horroud River. Appl Water Sci 7, 4725–4733 (2017). https://doi.org/10.1007/s13201-017-0635-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-017-0635-0