Abstract
New porous ceramics (PC) prepared by recycling waste glass bottle of soft drinks (80 mass%) and bamboo charcoal (20 mass%) without any binder was applied to the waste water purification under aeration at 25 °C. Artificial waste water (15 L) containing 10 mL of milk was examined by combining 15 mL of activated sludge and 750 g of PC. Biochemical oxygen demand (BOD) showed a marked decrease from 178 to 4.0 (±0.1) mg L−1 in 5 days and to 2.0 (±0.1) mg L−1 in 7 days, which was equal to the Environmental Standard for the river water (class A) in Japan. Similarly, chemical oxygen demand (COD) decreased from 158 to 3.6 (±0.1) mg L−1 in 5 days and to 2.2 (±0.1) mg L−1 in 9 days, which was less than the Environmental Standard for the Seawater (class B) in Japan: 3.0 mg L−1. These results prove the high water purification ability of the PC, which will be effectively utilized for the purification of drinking water, fish preserve water, fish farm water, etc.
Similar content being viewed by others
Introduction
About one million tons of glass bottles are discarded in Japan every year (Suzuki et al. 2006). It is generally known that the cost for the preparation of glass bottles by recycling waste glass is reduced to 70–80% of the cost using raw materials like Na2CO3, CaCO3, and SiO2. Another advantage of recycling waste glass is the reducing emission of CO2 during bottle fabrication. By recycling waste glass bottle of soft drinks (70–92 mass%) and wood charcoal (8–30 mass%) at 800 °C, new porous ceramics (PC) were invented (Nishida 2011). In the invention, wood charcoal was introduced in order to produce cross-linked 3D-channels in the molten phase of the sodalime silicate glass, the network of which was constituted of distorted SiO4 tetrahedra.
Related PC was previously invented by recycling coal ash (fly ash) and the waste glass bottle of soft drinks at 950–1150 °C (Nishida 2009, 2010; Tamaki et al. 2006). In this PC, coal ash composed of aluminosilicates was recycled as the main component (e.g. 70 mass%), and no additional carbon source was added to the powder mixture since the coal ash originally had several mass% of unburned carbon. Depending on the composition and mixing ratio of the raw materials and on the sintering temperature, diameters of the cross-linked 3D-channels varied from several nm to several μm, which was effective for the microbes to proliferate by digesting organic matters in the water.
During the preparation of PC, a lot of CO2 bubbles were formed in the molten glassy phase. They randomly connected to each other to form cross-linked 3D-channels in the PC. When the coal ash was recycled as the main component (Nishida 2009, 2010; Tamaki et al. 2006), cross-linked 3D-channels were formed in the molten phase of aluminosilicate glass, the network of which was constituted of distorted AlO4 and SiO4 tetrahedra. The optimal temperature for fabricating the cross-linked 3D-channel depended on the chemical composition and mixing ratio of the raw materials; the higher was the fraction of the coal ash containing a lot of Al2O3, the higher was the sintering temperature. This is not favorable from the view point of energy saving. It is expected that preparation of PC by recycling the waste glass which is free from heat-resistant Al2O3, as the main component, leads to a great advantage for the manufacturers and consumers. In the present study, water purification study was first carried out using the PC prepared by recycling waste glass bottle (main component) and bamboo charcoal at 800 °C, which was much lower than that required for recycling the coal ash as the main component and the waste glass bottle, i.e., 950–1150 °C (Nishida 2009, 2010; Tamaki et al. 2006).
Bamboo is naturally grown in several Asian countries. Since the bamboo grows so quickly in spring and summer seasons, “bamboo forest” spreads undesirably. Hence, bamboo cutting is required for the environmental preservation. Bamboo charcoal is prepared by burning dried bamboos in air. It is utilized as fuel, cleaner for the exhausted gas, deodorant, etc. Asada et al. reported that micropores of 0.5–2 nm sizes were effective for the physical adsorption of several hazardous gases like formaldehyde, toluene, benzene, ammonia, and indole. (Asada et al. 2002). Mesopores of 6.5 and 15 nm sizes were reported together with macropores of 60 and 450 nm sizes, and the pore size distribution depended on the temperatures for the bamboo charcoal preparation (500, 700, and 1000 °C).
It is generally known that fungi or bacteria are of μm sizes and play a principle role in water purification. In the present study, waste glass bottle of soft drinks and bamboo charcoal were recycled to prepare new PC having “macropores” with diameters more than several tens μm so that several microbes could easily proliferate therein. It is known that activated sludge (AS) contains a lot of aerobic fungi, bacteria, and protozoa, which are effectively utilized for sewage treatment. They can proliferate in the waste water by eating organic matters and oxidize toxic ammonia to nitrite or nitrate ions in the aerobic environment. Phosphorus components are effectively absorbed by the AS. Surplus sludge produced as a result of such biological activity should be regularly removed from the waste water treatment plants, and will be utilized as a fertilizer.
Very recently, volcanic ash-recycled PC was investigated (Nishida 2016; Ando et al. to be published). Since the volcanic ash was composed of heat-resistant aluminosilicates, the batch composed of ca. 70 mass% of volcanic ash and ca. 30 mass% of waste glass bottle prepared at 950 °C. Satisfactory sea water cleaning was confirmed with the artificial sea water in which several tropical fishes were actually living under aeration. Commercially available aerobic bacteria in the macropores of PC could decompose several organic matters like surplus feeds, excrements, urine, ammonia, and nitrite ions.
Experimental
In this study, new PC was prepared by sintering a powder mixture composed of 80 mass% of glass bottle of soft drinks (so-called sodalime silicate glass composed of 73.5 mass% of SiO2, 15.5 mass% of Na2O, and 11.0 mass% of CaO) and 20 mass% of commercially available bamboo charcoal at 800 °C for 60 min in air. Before the sintering, the waste glass was thoroughly pulverized in a ball mill for more than 1 day so that the grain size is less than 100 mesh. It was mixed with the powder of bamboo charcoal in a mortar. The powder mixture placed in an alumina box without any “binder” was sintered in an electric muffle furnace, the temperature of which was gradually increased from room temperature to 800 °C in 40 min. After heating for 60 min, the sintered sample was gradually cooled down to the room temperature in more than 4 h.
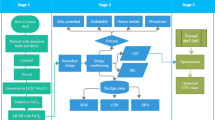
Experimental set-up for the water purification is illustrated in Fig. 1. Water purification test was conducted under aeration in a water tank in which pure water (15 L), milk (10 or 100 mL), and AS (15 mL) were circulated using a water pump. This experimental condition has commonly been applied to the waste water purification (Nishida 2009, 2010; Tamaki et al. 2006). It enables a direct comparison of the waste water purification abilities of different types of PCs. For evaluating the water purification effect, COD (chemical oxygen demand) and biochemical oxygen demand (BOD) were determined. The “Ca2+-rich milk” (Glico Dairy Products Company, Limited, Japan) had 8.9% of non-fat milk solid component and 2.5% of milk fat. A given amount of PC (750 g) was placed in a transparent plastic box in which the waste water was aerated all the time. Temperature of the water was fixed at 25 °C during the purification experiment.
COD was determined by the KMnO4 method using a conventional instrument: Quick COD Type HC-607 (CENTRAL KAGAKU CORP, Japan). BOD was determined using a conventional instrument: BOD Trak (CENTRAL KAGAKU CORP, Japan). Before the BOD measurement, sampling water (95 mL) was incubated at 20 °C for 5 days.
Results and discussion
Change of the transparency
Original artificial waste water was gray in color due to the milk and AS, as shown in Fig. 2 (top left). Gray color gradually disappeared during the water purification process. It became nearly transparent in 3 days (bottom left) and finally highly transparent in 5 days (bottom right). These photos demonstrate that the waste water could be successfully purified by the biological action of AS in association with the PC placed in the water tank. We can understand that the highly permeable PC enabled the microbes to proliferate and decompose the organic matters in the artificial waste water, since the PC could retain 60% of water of its own mass. In case of the comparative purification experiment of the solution “without the PC” (Fig. 3), the rate of decoloration was slower than that of the solution containing the PC (Fig. 2) by a few days. These results indicate that the AS played a principle role in the water purification. Figures 2 and 3 prove that the PC could effectively promote the biological action of the microbes which were proliferating in the biofilms of 3D-channels, and that the microbes could effectively decompose the protein, fat, minerals, etc., which were included in the artificial waste water.
Detailed characterization of the PC itself will be reported elsewhere together with additional experimental results. It is noted that the PC prepared by recycling the waste glass and bamboo charcoal had macropores with diameters of more than several tens μm, as confirmed by the observation under an optical microscope. Macropores of these sizes will be favorable for the microbes to proliferate therein.
Change of BOD
Change of BOD during the water purification is plotted in Fig. 4, in which red circles refer to the results obtained for the artificial waste solution containing 10 mL of milk, 15 mL of AS, and 750 g of PC. Figure 4 shows a marked decrease in BOD from 178 (±1) mg L−1 to 46, 28, 18, and 4.0 (±0.1) mg L−1 in 2, 3, 4, and 5 days, respectively. The BOD value of 4.0 (±0.1) mg L−1 corresponds to the purification rate of 97.8%. In 7–11 days, a constant BOD value of 2.0 (±0.1) mg L−1 was obtained, corresponding to the satisfactory purification rate of 98.9%. It is noted that the BOD of 2.0 mg L−1 is equal to the Environmental Standard for the River Water (class A) in Japan, which is suitable for the tap water (Japan 2017a).
Blue triangles were obtained from the comparative experiment using 10 mL of milk and 15 mL of AS “without the PC.” They showed a gradual decrease in BOD from 78 (±1) mg L−1 to 16, 20, 18, 14, and 20 (±1) mg L−1 in 2, 3, 4, 5, and 7 days, respectively. The BOD of 14 (±1) mg L−1 corresponds to the purification rate of 82%. It is noted that these BOD values are much larger than those obtained using PC (red circles), and that the PC plays a very effective role in the water purification.
Green circles plotted in Fig. 4 refer to the change of BOD obtained from the other experiment using highly concentrated artificial waste water containing 100 mL of milk, AS (15 mL), and PC (750 g). The BOD showed a marked decrease from 784 (±1) mg L−1 to 98, 50, 16, and 26 (±1) mg L−1 in 2, 3, 4, and 5 days, respectively. A purification rate of 96.7% was observed in 5 days, and finally the BOD decreased to 2.0 (±0.1) mg L−1, corresponding to the water purification rate of 99.7%. In the early water purification experiment using the PC prepared from waste glass (80 mass%) and wood charcoal (20 mass%), BOD changed from 360 to 2.3 mg L−1 in 15 days, corresponding to the purification rate of 99.4% (Nishida 2011). These results suggest that bamboo charcoal and wood coal could bring about almost the same purification effect when recycled to the PC.
Pink triangles were obtained from the comparative experiment using 100 mL of milk and AS (15 mL) “without the PC,” in which BOD showed a gradual decrease from 682 (±1) mg L−1 to 372, 148, 66, 60, and 56 (±1) mg L−1 in 2, 3, 4, 5, and 7 days, respectively. BOD of 60 mg L−1 corresponds to the purification rate of 91.2%. These values are larger than those obtained from the corresponding experiment using the PC (green circles), indicating that the PC could effectively contribute to the water purification.
Change of COD
Figure 5 depicts the change of COD values. Red circles obtained from the experiment using 10 mL of milk, AS (15 mL), and PC (750 g) showed a marked decrease from 158 (±1) mg L−1 to 30, 13, 6.9, and 3.6 (±0.1) mg L−1 in 2, 3, 4, and 5 days, respectively. The COD value of 3.6 mg L−1 corresponds to the purification rate of 97.7%, which is comparable to the result of BOD experiment described above (97.8%). The COD value finally decreased to identical values of 2.1–2.2 (±0.1) mg L−1 in 9–15 days, reflecting the purification rate of 98.6–98.7% which were also comparable to that observed from the BOD measurement described above (98.9%). It is noted that the COD values of 2.1–2.2 (±0.1) mg L−1 are less than the Environmental Standard for the Seawater (class B) in Japan (3.0 mg L−1; Japan 2017b).
Blue triangles were obtained from a comparative experiment using 10 mL of milk and AS (15 mL) “without the PC.” The COD showed a gradual decrease from 160 (±1) mg L−1 to 28, 15, 13, 11, and 10 (±1) mg L−1 in 2, 3, 4, 5, and 7 days, respectively. In 5 days, a purification rate of 93.1% was obtained. These results proved that the water purification was more successful when the PC was applied in combination with the AS.
Change of the COD measured for the artificial water containing 100 mL of milk, AS (15 mL), and PC (750 g) is also plotted in Fig. 5 with green circles, which showed a marked decrease from 491 (±1) to 110, 36, 22, and 32 (±1) mg L−1 in 2, 3, 4, and 5 days, respectively. A COD value of 32 mg L−1 obtained in 5 days reflects a purification rate of 93.5%. Finally the COD decreased to 7 (±0.1) mg L−1 in 15 days, corresponding to a purification rate of 98.6%. It is noteworthy that the application of the PC prepared from waste glass (80 mass%) and wood charcoal (20 mass%) to the corresponding water (Nishida 2011), changed COD from 388 to 4.2 mg L−1 in 15 days, corresponding to the purification rate of 98.9%. These results suggest that the bamboo charcoal and wood coal could bring about essentially the same purification effect when recycled to the PC. It is expected that they could compensate each other as the raw materials.
COD values measured from a comparative experiment using 100 mL of milk and AS (15 mL) “without the PC” (pink triangles; Fig. 5) showed a gradual decrease from 333 (±1) to 215, 105, 56, 47, and 40 (±1) mg L−1 in 2, 3, 4, 5, and 7 days, respectively. The COD value of 47 mg L−1 corresponds to the purification rate of 85.9%. These COD values revealed that water purification became distinguished when the PC was utilized in association with the AS, as confirmed from the measurement of BOD values (Fig. 4).
Conclusion
Water purification experiment using new PC prepared by recycling waste glass (main component) and bamboo charcoal showed a satisfactory result, showing a distinguished decrease in BOD and COD values especially when combined with AS. Bamboo charcoal was utilized in order to prepare 3D-channels in the molten phase of the sodalime silicate glass during the sample preparation. Several microbes proliferating in the biofilms of 3D-channels could play a principle role in the purification of artificial waste water. It is expected that the new PC will be effectively utilized for the purification of drinking water, fish preserve water, fish farm water, etc., just by circulating the water under aeration. It will also be effectively utilized as a biological filter at the household- or public-waste water treatment facilities. It is also expected that the PC will be utilized as functional materials like microbe carrier, cleaner for the exhausted gas, and heat insulation material.
References
Ando T, Fujita Y, Kakinaga M, Oka N, Nishida T Water purification using porous ceramics prepared by recycling volcanic ash and waste glass. Appl Water Sci (to be published)
Asada T, Ishihara S, Yamane T, Toba A, Yamada A, Oikawa K (2002) Science of bamboo charcoal: study on carbonizing temperature of bamboo charcoal and removal capacity of harmful gases. J Health Sci 48(6):473–479
Japan (2017a) Environmental Standard for the River Water in Japan. Ministry of the Environment (Japan). Available from http://www.env.go.jp/kijun/wt2-1-1.html (in Japanese). Accessed 03 Jan 2017
Japan (2017b) Environmental Standard for the Seawater in Japan. Ministry of the Environment (Japan). Available from http://www.env.go.jp/kijun/wt2-2.html (in Japanese). Accessed 03 Jan 2017
Nishida T (2009) Japanese Patent: No. 4269011
Nishida T (2010) Science education combined with the invention and innovation of materials science. Res Rep Kinki Univ (Kayanomori) 13:7–12 http://id.nii.ac.jp/1391/00011463/ (in Japanese)
Nishida T (2011) Japanese Patent: No. 4817367
Nishida T (2016) Japanese Patent: No. 5959832
Suzuki H, Itasaka Y, Makino K (2006) Heat and mechanical properties of waste glass aggregate as a base course material for taking measures of heat island. Jpn Geotech J 1(3):85–93 (in Japanese)
Tamaki J, Kubuki S, Nishida T (2006) Water purification with porous ceramics. Res Rep Kinki Univ (Kayanomori) 5:7–12 http://id.nii.ac.jp/1391/00015006/ (in Japanese)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nishida, T., Morimoto, A., Yamamoto, Y. et al. Waste water purification using new porous ceramics prepared by recycling waste glass and bamboo charcoal. Appl Water Sci 7, 4281–4286 (2017). https://doi.org/10.1007/s13201-017-0561-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-017-0561-1