Abstract
Thirty-nine (23 dug and 16 tube well) samples were geochemically evaluated and investigated to ascertain the quality of water in Khipro, Sindh. The analytical results exhibited abundance of major cations and anions in Na+ > Ca2+ > Mg2+ > K+ and Cl− > HCO3 − > SO4 2− sequence. Stiff diagram showed dug well sample have high Na–Cl and moderate Mg–SO4 content as compared to tube well samples. Majority of dug well samples appeared as Na–Cl type on Piper diagram while tube well samples are mixed type. Gibbs diagram reflected evaporation as a dominant phenomenon in dug well; however, tube well samples are declined toward rock dominance. Process of ion exchange was witnessed from Na+ versus Cl− and Ca2+ + Mg2+ versus HCO3 − + SO4 2− plots. Principal component analysis also discriminates dug well and tube well water by means of positive and negative loading based on physical and chemical composition of the groundwater. Studied and computed parameters like pH, EC, TDS, TH, Na+, K+, Ca2+, Mg2+, Cl−, SO4 2−, HCO3 −, sodium adsorption ratio, magnesium adsorption ratio, potential salinity, residual sodium carbonate, Na%, Kelly’s ratio, and permeability index were compared with WHO to evaluate studied water for drinking and agricultural purposes. Except Na+ and K+, all chemical constrains are within the allowed limits, set by WHO for drinking water. Similarly, most of the groundwater is moderately suitable for irrigation uses, with few exceptions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is a natural resource and a basic need for drinking, domestic, agricultural, industrial, environmental activities, etc. (Prasanth et al. 2012). Due to rapid increase in world’s population, the availability of water becomes scarce and many of the countries in South Asia, Middle East and Africa would face water crisis. Supply of safe drinking water is an ignored sector in Pakistan (Kausar et al. 2011). Huge number of people (70 %) in Pakistan lives in rural area where, safe drinking water is not available to them. Sindh is facing a severe shortage of water since few decades and demand of water in Sindh is high due to arid climate, extreme temperatures, high evaporation, and low precipitation.
Geochemistry and quality evaluation of water depends upon various physicochemical factors, mobility of elements, and climate (Bashir et al. 2013). Ion exchange mechanism between groundwater and composition of aquifer is also accountable for the occurrence of ions in water (Aghazadeh and Mogaddam 2010). Study of fundamental processes and factors that affect groundwater quality is necessary in managing significant resource (Sundaram et al. 2009).
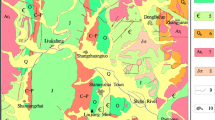
The study area lies in eastern side of the Khipro, Sanghar District, Sindh, Pakistan (Fig. 1) and is comprised of the desert, commonly known as Acchro Thar. The western part of Khipro area is irrigated by Nara Canal, whereas the study area is dry and seepage from canal is the main source of water. The whole area is covered by alluvial sediments consists mainly of fine to medium sands, silts, and clays of Sub-recent to Recent age over the Tertiary rocks. The nature of the soils varies considerably from place to place; sand and clay belts spread throughout the area. Sand belts are wider in extent than the heavy soil belts.
The objective of this study include identification of water types, origin, and geochemical processes influencing the composition of water by multivariate statistical methods such as correlation matrix and principal component analysis (PCA). Important parameters of water like pH, Ca, Mg, Na, K, Cl, SO4, HCO3, CO3, TH, and TDS were assessed for drinking water quality with reference to WHO standards. Significant irrigational parameters such as SAR, Na%, PS, RSC, MAR, KR, and PI are also planned to evaluate. Possibly the present findings will help to accomplish practical guidelines for combating drinking and agriculture problems faced by the inhabitants of area.
Materials and methodology
Thirty-Nine (39) random groundwater samples (23 dug well and 16 tube well) from Khipro, Sanghar District, Sindh, were collected. Sample sites were plotted on the satellite image using ArcGIS 10 software (Fig. 1). Sample collection were made in 1.5 liter capacity prewashed polyethylene bottles (Akoto and Adiyiah 2007) after allowing tube wells to flow for at least 10 min to get representative sample of the groundwater (Tahir et al. 2010), whereas dug well samples were collected with the help of bucket. Standard methods of American Public Health Association (APHA 2012) were used to analyze chemical and physical characteristics of water. In situ measurement of TDS, EC and pH were done using Denver Instrument Model 50. Sulfate was estimated by gravimetric method, while Cl, HCO3, Ca and Mg were estimated volumetrically. Alkalis (Na, K, and Li) were measured with the help of flame photometer (FGA-350-L Gallen Kamp). Standards were used to calibrate the instrument. Software (SPSS_20 for Windows) was applied for the appraisal of PCA.
Results and discussion
Ionic composition
The data of dug and tube well samples, their statistical analysis, and Ionic balance Error (IBE) are presented in Table 1. Analytical data of present study indicate that IBE is within acceptable limit of 5 % (Kumar and Khan 2015). Concentration of major ions in the dug well sample is higher than tube well, except Ca. El-Fiky (2009) is used Schoeller diagram to compare major ionic composition of studied samples. Plots of average composition of present study display trends as Na > Ca > Mg > K and Cl > HCO3 > SO4 for cations and anions, respectively (Fig. 2). Average composition on stiff diagram signifies supremacy of Na–Cl along with Mg–SO4 as second dominant pair in dug well samples (Fig. 2). However, in tube wells, 69 % samples shows dominance of Na–Cl while 31 % samples are Ca–HCO3, reflecting fluctuating geochemical environment for the distribution of ions in the study area. The shape of polygon of stiff diagram also classifies the water of different origin (Nadiri et al. 2013; Hounslow 1995). In studied samples, the possible sources of origin are ion exchange, saline, and mixed type.
Hydro-geochemical facies
Chemical composition of samples are plotted on Piper diagram to infer type of hydrofacies, mixing of water from different sources, sulfate reduction, saline water, and other-related hydrochemical problems (Herojeet et al. 2013; Prasad et al. 2009). In Piper diagram (Fig. 3), each of the two cations and anion triangles are categorized into four fields illustrating water of different types. Among cations samples are distributed in zone B (mixed type) and zone D (Na–K type in the cation triangle), while in anion triangle samples are plotted in zone B and zone G (Cl type) except only one sample showing influence of HCO3-type water (Fig. 3). The diamond shape is divided into nine zones (Fig. 3) to differentiate groundwater of diverse origin (Chowdhury and Gupta 2011; Sadashivaiah et al. 2008). Most of the samples are plotted in zone 7 which suggests Na–Cl type of water (saline nature) with the greater influence of Na + K as compared to Ca + Mg (zone 2), whereas the remaining samples are plotted in zone 9 which suggests mixed type of water with the dominance of Ca + Mg over Na + K (zone 1). On contrary, all collected samples were plotted in (zone 4) demonstrates excess of SO4 + Cl over CO3 + HCO3 (Fig. 3). Current study demonstrates close geological environment of ionic distribution. Majority of studied samples are clustered in Na + K-Cl + SO4 facies, which can be attributed to saline water influence. This might be due to the presence of salt lakes in the surrounding areas, formed in consequence of evaporation.
Geochemical processes
Gibbs (1970) proposed diagrams based on ratios of Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3 −) versus TDS, to comprehend main processes (evaporation, precipitation and rock weathering) which control the chemistry of the groundwater. Nearly all studied samples are confined to evaporation domain, indicating that the composition of dug and tube well water of Khipro region is mainly controlled by evaporation process in consequence of the hot and arid climate prevailing in the region (Fig. 4). Few samples also show partial influence of rock weathering (Fig. 4) in the area.
Mechanisms controlling groundwater quality in Khipro area (after Gibbs 1970)
The Na versus Cl and Ca + Mg versus HCO3 + SO4 mutual plots are used to indicate the effect of ion exchange (HAP 2011). Bivariate plot Na versus Cl reveals relatively higher concentrations of Na compared to Cl for majority of the groundwater samples, exhibiting that most of the Na is acquired along groundwater flow paths without adding Cl (Fig. 5a). Majority of the dug and tube well samples are plotting on or above the 1:1 Na/Cl line, indicating Na–Cl type water. The higher proportion of HCO3 and SO4 compared to Ca and Mg (Fig. 5b), also suggests the role of ion exchange. Similarly, the increase of the Na/Ca ratio as TDS increases provides strong evidence of the replacement of Ca by Na as groundwater percolate farther along flow paths (which corresponds to increase TDS).
According to Hounslow (1995), geological processes and source rock are also responsible for the variation in ionic compositions of waters based upon Na and Cl relationship. Water having Na+/(Na+ + Cl−) ratio <0.5 reveals reverse softening, while >0.5 suggests that water is the result of ion exchange. In the study area, majority of samples (i.e., 14 out of 23 dug well and 11 out of 16 tube wells) have ratio >0.5 which indicates prevalence of ion exchange, where Na is replacing Ca from the aquifer material (clay minerals). However, in few cases, reverse ion exchange occur where Ca is replacing the Na (natural softening).
Statistical analyses
Correlation matrix
Nesrine et al. (2015) and Batabyal (2014) used correlation matrix to measure the degree of closeness among the different variables. The correlation matrix of 12 quality parameters, for the dug well and tube well samples, is presented in Table 2. In all the studied samples, there is a very strong positive correlation (0.9–0.99) between EC and Cl, Na; TDS and Na, Cl, EC; Cl and Na. Very strong correlation between TH and Mg also exists in dug well samples while SO4 and TDS, EC relationship is strong in tube well samples. These parameters contribute to groundwater salinization (Nesrine et al. 2015). Very strong correlation of Na and Cl reflect their genetic association may be due to evaporation.
A strong to fairly moderate correlation (0.89–0.71) occurs between TH and Ca; SO4 and Mg; K and Li in all samples. However, SO4 and TDS, EC, TH in dug wells, and TH with Mg, K; SO4 and Na, Cl in tube well samples also revealed strong to fairly moderate correlation matrix (Table 2). Strong correlation of Ca, Mg, and SO4 with TH indicates that TH is essentially a permanent hardness. The moderate to poor correlation among Ca and SO4 indicates that there is no gypsum dissolution but the excess of Ca could be due to the carbonate and cation exchange (Zhang et al. 2014).
Among dug well samples, moderate correlation exist between Mg and TDS, EC and Ca; TH and TDS, EC. Similarly, tube well samples display moderate correlation between HCO3 and TDS, EC and Na; Mg and EC, K and Li; Ca and K (Table 2). Perhaps the positive correlation between Ca and K is due to ion exchange from clay minerals. The pH of dug well samples exhibits weak negative correlation matrix with all other parameters except Na and HCO3.
Principal component analysis (PCA)
PCA is an efficient tool to discriminate patterns in groundwater chemistry from huge physical and chemical analytical data (Kiymaz and Karadavut 2014; Nagaraju et al. 2014; Okiongbo and Douglas 2015). Factor analysis is one of the significant multivariate techniques that perform through PCA method. Based on rotated components matrix three factors have been extracted for analysis of major elements association in the studied samples (Fig. 6a, b). The first factor accounts for 31.55 and 42.96 % of the variance in dug and tube well samples, respectively, showing very high-positive loadings for Na, TDS, EC, and Cl while moderate loadings have been noticed for SO4. Depth mainly controls anions (SO4, HCO3) along with Na+ in deep tube wells; however, its role in shallow dug wells is independent (Fig. 6b). Second factor explained 26.40 and 30.37 % of the total variance, respectively. The rotated factor matrix shows that the highest positive loading has been recorded for TH (0.91), Ca (0.89), Mg (0.82), and SO4 (0.68), while negative loading for HCO3 (−0.52) among dug well samples (Fig. 6a). High-positive loading has been recorded for TH (0.92), K (0.87), Li (0.79), Ca (0.77), and Mg (0.76) in the tube well samples. The HCO3 shows reverse (positive) trend with Ca and Mg in tube well samples. The third factor for dug wells (Fig. 6a), amplifying 16.28 % of the total variance, shows high-positive loadings for Li (0.95) and K (0.94), probably this relation is in good agreement with granites as source rock. Similarly, 10.34 % third factor variance shows high-positive loadings of pH (0.78) with Na, Cl and SO4 in tube well, and negative loading for HCO3 (−0.57); however, pH in dug wells shows independent character.
Drinking water quality
Drinking water availability is an essential requirement of humans which is supported by international law and the declarations (Kausar et al. 2011). In drinking water, pH is one of the most significant quality parameters and may affect health of humans (Rahmanian et al. 2015). The pH values (Table 1) indicate that all samples are alkaline type and safe for drinking uses, as suggested by WHO (2004). The TDS values of studied samples revealed only three tube well samples are in desirable limit (<500). Nearly 12 dug well (52 %) and 7 tube well samples (44 %) are found within WHO (2004) limit (500–1500); however, the remaining samples from dug well (11) and tube well (6) are above the maximum allowable limit (>1500). It indicates presence of slightly higher concentration of salts and related problems such as hardness. The mean value of EC 2.62 and 1.98 dS/m in dug wells and tube wells, respectively, indicates that the groundwater samples have values higher than WHO desirable limit (1.40 dS/m). In contrast, Herojeet et al. (2013) considered that groundwater samples having EC values less than 2.00 dS/m as fresh water and are fit for domestic as well as drinking purposes. Total hardness of majority of samples was less than the WHO guideline value of 500 mg/l as CaCO3 (WHO 2011). Only six dug well and two tube wells samples show hardness higher than maximum allowable limit for drinking purpose (500).
The mean values of Na and K in the groundwater of the study area are higher than the maximum permissible limit (Fig. 2) except in few samples which were found within the maximum allowable limit. The average concentrations of Ca, Mg, SO4, and Cl in the study area are much lower than the WHO standard for drinking water (Fig. 2). The mean value of HCO3 ions of tube well samples (Table 1) is less than desirable limit (300 mg/l), and the average value of dug well samples is less than maximum permissible limit (600 mg/l). Majority of studied samples are considered safe for drinking purpose.
Irrigation water quality
pH
Crops are very sensitive to pH of the irrigating water. The best range of pH for irrigation is between 6.5 and 8.4 (Bauder et al. 2010) indicating all studied samples are suitable for irrigation purpose (Table 1) except one dug well sample# AS (pH 8.5) which is slightly higher than pH range for irrigation water.
Salinity hazard
Salinity hazard is the leading water quality character which affects the production of crops, measured in terms of EC. In case of high EC, low amount of water is available to plants (Bauder et al. 2013). USDA Salinity Laboratory classifies irrigation water into four salinity classes on the basis of electrical conductivity and total dissolve solids. Studied samples indicate high to very high salinity hazard except three tube well samples which are found in moderate range.
Potential salinity (PS)
Beside EC, PS also controls the suitability of water for irrigation (Ogunfowokan et al. 2013). Low soluble salts accumulate in the soil is beneficial for irrigation, while high soluble salts increase the salinity of the soil (Siamak and Srikantaswamy 2009). It is calculated as PS = Cl− + ½SO4. The mean PS of the study area is 18.28 and 12.47 meq/l (Table 1) for dug wells and tube wells, respectively.
Sodium adsorption ratio (SAR)
High concentration of cations is mainly responsible for sodium or alkali hazard in irrigation water (Ogunfowokan et al. 2013; Gholami and Srikantaswamy 2009). Low SAR values are always desirable (Raihan and Alam 2008) because it influence infiltration rate of water. It can be determined from the following expression:
where Na, Ca and Mg are in meq/l.
The calculated SAR values indicate that 87 % dug well and 92 % tube well samples are good to excellent for irrigation in the area.
Sodium percent (Na %)
The amount of sodium in irrigation water is referred as Na%. The Na content of water reacts with the soil and accumulates in the pore spaces thus reducing its permeability (Khan and Abbasi 2013). Deterioration of soil structure, poor infiltration, and low aeration is caused due to high Na% (>60 %) in agriculture water supply. Sodium percent is determined using the equation of Sadashivaiah et al. (2008) and Prasad et al. (2009) as:
The assessed amount of Na% revealed that tube wells are relatively more suitable than dug well samples. Most of the dug well showed Na% greater than 60 % which may cause salt accumulation in the soil during repeated irrigation.
Plot of Electrical conductivity verses Na% is important to evaluate the irrigation water suitability (Khan and Abbasi 2013; Khodapanah et al. 2009). The binary plot consists of five distinct agriculture water quality fields (Fig. 7a). Adopting this scheme, studied samples are found in permissible to excellent range while few are unsuitable for irrigation purpose (Fig. 7a). Similarly, Wilcox diagram is also used to classify water for irrigation (Ramkumar et al. 2010). Water samples of present study are plotted in the area of S1C2, S1C3, S1C4, and S2C3 indicating low SAR and medium to high salinity (Fig. 7b). It is moderately suitable for irrigation purposes; however, rest of samples occupy fields of S2C4, S3C3, S3C4, and S4C4 reflecting high hazard water-type (Fig. 7b).
Residue sodium carbonate (RSC)
Hazard of CO3 and HCO3 on the quality of agricultural water is determined as Residual sodium carbonate (Khan and Abbasi 2013). It can be calculated from the expression as:
According to RSC values, irrigation water is classified into three classes: safe, marginal, and unsuitable (Camberato 2001). Data showed that bulk of studied waters are appropriate for irrigation. In 5 out of 23 dug wells and 2 out of 16 tube well samples, RSC value exceeds 2.5 meq/l, these waters are considered unsuitable and harmful for irrigation. According to Aghazadeh and Mogaddam (2010), high RSC restrict movement of water and air in soil.
Magnesium adsorption ratio (MAR)
The MAR is also one of the considerable factors to evaluate quality of irrigation water. It is assessed as follows:
Gupta and Gupta (1987) mentioned that high MAR affects the soil unfavorably, a harmful effect on soils appear when MAR exceeds 50. In the present study, 66 and 30 % tube well and dug well samples, respectively, had MAR less than 50 which cause no harm to soil and the rest were above 50 which might harmful to soil.
Kelley’s ratio (KR)
Kelly (1963) described a ratio Na/Ca + Mg which evaluate suitability of water for irrigation purposes. Kelley’s ratio should not exceed unity for irrigation water. Among tube wells, 66 % samples have KR values <1 while in dug well samples only 13 % waters (KR < 1) are suitable for irrigational uses. Rest of water samples have KR values greater than unity. The average ratio is greater than unity in both dug well and tube well samples (Table 1).
Permeability index (PI)
Soil permeability is reduced by the consecutive use of water-containing high salts (Singh and Singh 2008). The PI is employed to assess quality of irrigation water and can be computed from the equation of Doneen (1962) as:
According to this classification, irrigation water with high permeability (>75 %) is classified as Class I, while Class II has permeability between 75 and 50 % (Nagaraju et al. 2006). Class I and Class II waters are categorized as good for irrigation purpose. The third category (Class III) is unsuitable with 25 % of maximum permeability (Dhirendra et al. 2009). On the basis of PI classification, majority of water samples of the study area belong to class I and II (Fig. 7c), and are of good irrigation quality except few samples.
Conclusions
Geochemical studies of groundwater of Khipro, Sanghar District, Sindh display a trend of Cl > HCO3 > SO4 for anions and Na > Ca > Mg > K trend of cations. Stiff diagrams of the studied samples reveal Na–Cl as major ions pair accompanied with Mg–SO4 in dug well samples and Ca–HCO3 in tube wells. Shape analysis of Stiff patterns demonstrates ion exchange, saline, and mixed type of origin. Piper diagram also reveals majority of dug well and few tube well samples are of Na–Cl type while remaining samples are of mixed type of water. Plots of studied samples on Gibbs’ diagrams, divulge evaporation as dominant process with minor impact of rock wreathing on few samples. The Na versus Cl, Ca + Mg versus HCO3 + SO4, Na/Ca versus TDS, and Na+/(Na++Cl−) ratio indicates the effect of ion exchange. Strong correlation matrix between Na and Cl specify evaporation as one of the dominant process controlling water chemistry. Similarly, Ca–K relation specifies ion exchange process from clay minerals. Rotated components matrix diagram (PCA) shows close association among K–Li, it is a good agreement to show affiliation with the granitic rock and influence of evaporation and ion exchange. Average quantities of major ions in studied samples are found within maximum permissible limits except Na and K, which is higher than WHO standards. The average concentration of Mg, Cl, SO4, and HCO3 are close to desirable limits.
Electrical conductivity, SAR, Na%, RSC, MAR, KR, and PI values exhibit majority of the water samples from dug and tube wells are moderately fit for irrigation purpose except a few samples. On SAR versus EC plot, samples indicate high salinity-low sodium to high hazard water-type.
References
Aghazadeh N, Mogaddam AA (2010) Assessment of groundwater quality and its suitability for drinking and agricultural uses in the Oshnavieh Area, Northwest of Iran. J Environ Protect 1:30–40
Akoto O, Adiyiah J (2007) Chemical analysis of drinking water from some communities in the Brong Ahafo region. Int J Environ Sci Tech 4(2):211–214
APHA (American Public Health Association), AWWA (American Water Works Association), WEF (Water Environment Federation) (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, American Water Works Association and Water Environment Federation, Washington, DC. ISBN 978-087553-013-0
Bashir E, Naseem S, Hanif H, Pirzada T (2013) Geochemical study of groundwater of Uthal and Bela areas, Balochistan and its appraisal for drinking and irrigation water quality. Int J Agric Environ 2:1–13
Batabyal AK (2014) Correlation and multiple linear regression analysis of groundwater quality data of Bardhaman District, West Bengal, India. Int J Res Chem Environ 4(4):42–51
Bauder TA, Waskom RM, Davis JG (2010) Irrigation water quality criteria, Colorado State University Extension. http://www.ext.colostate.edu/pubs/crops/00506.html
Bauder TA, Waskom RM, Sutherland PL, Davis JG (2013) Irrigation water quality criteria. Colorado State University Extension Report # 0.506
Camberato J (2001) Irrigation water quality. Turfgrass Program, Clemson University; (Update from the 2001 Carolinas GCSA Annual Meeting). www.clemson.edu/turfornamental/
Chowdhury AK, Gupta S (2011) Evaluation of water quality, hydro-geochemistry of confined and unconfined aquifers and irrigation water quality in Digha Coast of West Bengal, India (A case study). Int J Environ Sci 2(2):576–589
Dhirendra MJ, Kumar A, Agrawal N (2009) Assessment of the irrigation water quality of river Ganga in Haridwar District. Rasayan J Chem 2:285–292
Doneen LD (1962) The influence of crop and soil on percolating water. In: Proceedings of the 1961 biennial conference on ground water recharge, pp 156–163
El-Fiky AA (2009) Hydrogeochemistry and geothermometry of thermal groundwater from the Gulf of Suez Region, Egypt. JKAU Earth Sci 20(2):71–96
Gholami S, Srikantaswamy S (2009) Analysis of agricultural impact on the Cauvery River water around KRS Dam. World Appl Sci J 6(8):1157–1169
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Gupta SK, Gupta IC (1987) Management of saline soils and water. Oxford and IBM Publ Co, New Delhi
HAP (Hydrogeological Assessment Project) (2011) Final technical report: Hydrogeological Assessment Project of the Northern Regions of Ghana 1:236
Herojeet RK, Rishi MS, Sidhu N (2013) Hydrochemical characterization, classification and evaluation of groundwater regime in Sirsa Watershed, Nalagarh Valley, Himachal Pradesh, India. Civil Environ Res 3(7):47–57
Hounslow AW (1995) Water quality data: analysis and interpretation. Lewis Pub, New York
Kausar S, Asghar K, Anwar SM, Shaukat F, Kausar R (2011) Factors affecting drinking water quality and human health at household level in Punjab, Pakistan. Pak J Life Soc Sci 9(1):33–37
Kelly WP (1963) Use of saline irrigation water. Soil Sci 95:355–391
Khan TA, Abbasi MA (2013) Synthesis of parameters used to check the suitability of water for irrigation purposes. Int J Environ Sci 3(6):2031–2038
Khodapanah L, Sulaiman WNA, Khodapanah N (2009) Groundwater quality assessment for different purposes in Eshtehard District, Tehran, Iran. Eur J Sci Res 36(4):543–553
Kiymaz S, Karadavut U (2014) Application of multivariate statistical analysis in the assessment of surface water quality in Seyfe Lake, Turkey. J Agric Sci 20:152–163
Kumar MS, Khan FLA (2015) Analysis of groundwater quality of some selected stations of Palar Riverbed in Vellore district, Tamilnadu, India. Int J Adv Sci Tech Res 2(5): ISSN 2249-9954. Available online on http://www.rspublication.com/ijst/index.html
Nadiri AA, Moghaddam AA, Tsai FT-C, Fijani E (2013) Hydrogeochemical analysis for Tasuj plain aquifer, Iran. J Earth Syst Sci 122(4):1091–1105
Nagaraju A, Suresh S, Killham K, Hudson-Edwards K (2006) Hydrogeochemistry of waters of mangampeta barite mining area, Cuddapah Basin, Andhra Pradesh, India. Turk J Eng Environ Sci 30:203–219
Nagaraju A, Sunil Kumar SK, Thejaswi A, Sharifi Z (2014) Statistical analysis of the hydrogeochemical evolution of groundwater in the Rangampeta area, Chittoor District, Andhra Pradesh, South India. Am J Water Resour 2(3):63–70
Nesrine N, Rachida B, Ahmed R (2015) Multivariate statistical analysis of saline water—a case study: Sabkha Oum LeKhialate (Tunisia). Int J Environ Sci Dev 6(1):40–43
Ogunfowokan AO, Ogunkoya OO, Obisanya JF (2013) Salinity and sodium hazards of three streams of different agricultural land use systems in Ile-Ife, Nigeria. Appl Water Sci 3:19–28
Okiongbo KS, Douglas RK (2015) Evaluation of major factors influencing the geochemistry of groundwater using graphical and multivariate statistical methods in Yenagoa city, Southern Nigeria. Appl Water Sci 5(1):27–37
Piper AM (1953) A graphic procedure I the geo-chemical interpretation of water analysis. USGS Groundw. Note no 12
Prasad DSR, Sadashivaiah C, Rangnna G (2009) Hydrochemical characteristics and evaluation of groundwater quality of Tumkur Amanikere Lake Watershed, Karnataka, India. J Chem 6(S1):S211–S218. http://www.e-journals.net
Prasanth SVS, Magesh NS, Jitheshlal KV, Chandrasekar N, Gangadhar K (2012) Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal stretch of Alappuzha District, Kerala, India. Appl Water Sci 2(3):165–175
Rahmanian N, Ali SHB, Homayoonfard M, Ali NJ, Rehan M, Sadef Y, Nizami AS (2015) Analysis of physiochemical parameters to evaluate the drinking water quality in the State of Perak, Malaysia. J Chem. Article ID 716125. doi:10.1155/2015/716125
Raihan F, Alam JB (2008) Assessment of groundwater quality in Sunamganj of Bangladesh. Iran J Environ Health Sci Eng 5(3):155–166
Ramkumar T, Venkatramanan S, Mary IA, Tamilselvi M, Ramesh G (2010) Hydrogeochemical quality of groundwater in Vedaraniyam Town, TamilNadu, India. Res J Environ Earth Sci 2(1):44–48
Sadashivaiah C, Ramakrishnaiah CR, Ranganna G (2008) Hydrochemical analysis and evaluation of groundwater quality in Tumkur Taluk, Karnataka State, India. Int J Environ Res Public Health 5(3):158–164
Siamak G, Srikantaswamy S (2009) Analysis of agricultural impact on the Cauvery river water around KRS dam. World Appl Sci J 6(8):1157–1169
Singh V, Singh UC (2008) Assessment of groundwater quality of parts of Gwalior (India) for agricultural purposes. Indian J Sci Technol 1(4):1–5
Sundaram B, Feitz AJ, Patrice de Caritat, Plazinska A, Brodie R, Coram J, Ransley T (2009) Groundwater sampling and analysis—a field guide. Geoscience Australia, Record 2009/27
Tahir MA, Rasheed H, Imran S (2010) Water Quality status in rural areas of Pakistan. Pakistan Council of Research in Water Resources (PCRWR). Publication No. 143-2010
WHO (2004) Guidelines for drinking-water quality, 3rd edn., 1WHO, Geneva, pp 143–220
WHO (2011) Guidelines for drinking water quality. WHO, Geneva
Wilcox LV (1955) Classification and use of irrigation waters. U.S. Dept. Agriculture Circular
Zhang X, Qian H, Chen J, Qiao L (2014) Assessment of groundwater chemistry and status in a heavily used semi-arid region with multivariate statistical analysis. Water 6:2212–2232
Acknowledgments
The Dean, Faculty of Science, University of Karachi is acknowledged for financial support. Mr. Atta Chaniho, CEO, Malhar Development Organization District Sanghar, Sindh Pakistan for his support during the fieldwork and hospitality at Khipro.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bashir, E., Huda, Su., Naseem, S. et al. Geochemistry and quality parameters of dug and tube well water of Khipro, District Sanghar, Sindh, Pakistan. Appl Water Sci 7, 1645–1655 (2017). https://doi.org/10.1007/s13201-015-0316-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-015-0316-9