Abstract
Mg−Al oxide, obtained by the thermal decomposition of a CO3 2−-intercalated Mg−Al layered double hydroxide (CO3·Mg−Al LDH), simultaneously absorbed Cl− and SO4 2− from seawater and generated a Mg−Al LDH intercalated with Cl− and SO4 2−. The Mg−Al oxide with a molar ratio Mg/Al = 4 was more superior than the oxide with Mg/Al = 2 for Cl− removal, whereas a reverse phenomenon was observed for SO4 2− removal. The removal of Cl− and SO4 2− by the Mg−Al oxide with Mg/Al = 4 could be represented by first-order and pseudo second-order reactions, respectively. The removal of both Cl− and SO4 2− by the Mg−Al oxide with Mg/Al = 2 could be represented by a pseudo second-order reaction. The removal of both Cl− and SO4 2− by the Mg−Al oxides with Mg/Al = 4 and 2 was proceeded under chemical reaction control. The adsorption isotherms for Cl− and SO4 2− adsorbed by the Mg−Al oxides could be expressed by Langmuir-type adsorption. These reactions were derived from monolayer adsorption, indicating the intercalation of Cl− and SO4 2− in the interlayer space of Mg−Al LDH. The uptake of Cl− and SO4 2− from seawater by Mg−Al oxide was proceeded spontaneously.
Similar content being viewed by others
Introduction
Salt damage can occur in agricultural lands after the incursion of seawater due to typhoons, storm surges, and tsunamis. It may result from land subsidence, penetration of seawater into groundwater, and leaching of saline from landfill sites. Treatment methods for correcting salt damage include flushing of agricultural land and the dilution of leachate with water. However, these methods require increased water consumption. Therefore, new treatment methods must be developed to remediate environmental salt damage.
Mg−Al layered double hydroxides (Mg−Al LDHs) are well known for their anion-exchange properties (Miyata 1983). Mg−Al LDHs can be represented by the formula [Mg 2+1-x Al 3+ x (OH)2] (An−) x/n ·mH2O, where x denotes the Al/(Mg+Al) molar ratio (0.20 ≤ x ≤ 0.33), and An− is an n-valent anion (Ingram and Taylor 1967; Allmann 1968). The structure of a Mg−Al LDH consists of a stack of Al-bearing, brucite-like octahedral layers, which are positively charged owing to the replacement of some Mg2+ with Al3+, and are electrically neutralized by interlayer anions. The interlayer spaces that are not occupied by anions can contain water molecules. The CO3 2−-intercalated Mg−Al LDH (CO3·Mg−Al LDH) can be converted into Mg−Al oxide by calcination at 450−800 °C:
This Mg−Al oxide can rehydrate and combine with anions to reconstruct the LDH structure:
Mg−Al LDH and Mg−Al oxide have received considerable attention as potential adsorbents for wastewater treatment. Their uptake of contaminants from aqueous solutions has been studied; these include reactive brilliant orange X-GN, arsenate, arsenite, fluoride, bromate, bromide, selenate, borate, nitrate, and chromate (Chubar 2011; Wu et al. 2011; Lv et al. 2012; Halajnia et al. 2012; Yu et al. 2012). As expressed in Eq. 2, the rehydration and subsequent combination of Mg−Al oxide with anions in solution is accompanied by the release of OH−. Based on this behavior, Mg−Al oxide could both neutralize and fix Cl− in the treatment of HCl (Kameda et al. 2000, 2002, 2005, 2006). Mg−Al oxide was also shown to treat mineral acids such as H3PO4, H2SO4, and HNO3 (Kameda et al. 2003).
As salt damage is typically derived from the Cl− and SO4 2− anions contained in seawater, the absorption capabilities of Mg−Al oxide for those species make it suitable for the treatment of seawater. We propose to spray Mg−Al oxide onto agricultural land as a treatment method for salt damage. In this study, the potential for Mg−Al oxide to absorb Cl− and SO4 2− simultaneously from seawater was examined with respect to time, temperature, and quantity of Mg−Al oxide. Kinetics and equilibrium studies on the treatment of seawater by Mg−Al oxide were conducted.
Materials and methods
All reagents were purchased from Kanto Chemical Co., Inc. CO3·Mg−Al LDH was prepared by the co-precipitation method. A Mg−Al solution ([Mg2+]+[Al3+] = 0.5 M, [Mg2+]/[Al3+] = 4.0 or 2.0) was prepared by dissolving Mg(NO3)2·6H2O and Al(NO3)3·9H2O in deionized water (500 mL). The Mg−Al solution was added dropwise to Na2CO3 solution (0.1 or 0.17 M, 500 mL) at 30 °C with mild agitation. The solution pH was adjusted to 10.5 by adding 1.25 M NaOH solution. The mixture was then stirred continuously at 30 °C for 1 h. The CO3·Mg−Al LDH product was isolated by filtering the resulting suspension, washing it thoroughly with deionized water, and drying under reduced pressure (133 Pa) at 40 °C for 40 h. Mg−Al oxide was then prepared by the thermal decomposition of CO3·Mg−Al LDH at 500 °C for 2 h. The Mg−Al oxide prepared from the Mg−Al solution with [Mg2+]/[Al3+] = 4.0 contained 41.5 wt% Mg and 11.7 wt% Al, resulting in a Mg/Al molar ratio of 3.9. This material was designated Mg−Al oxide (Mg/Al = 4). The Mg−Al oxide prepared from the Mg−Al solution with [Mg2+]/[Al3+] = 2.0 contained 20.6 wt% Mg and 11.6 wt% Al, resulting in a Mg/Al molar ratio of 2.0. This material was designated Mg−Al oxide (Mg/Al = 2).
The seawater contained 15,951 mg/L Cl−, 2,265 mg/L SO4 2−, 9,893 mg/L Na+, 360 mg/L K+, 1,054 mg/L Mg2+, and 310 mg/L Ca2+. Seawater (20 mL) and predetermined amounts of the Mg−Al oxides were placed in 50 mL screw-top tubes and shaken at 10−60 °C for 2 min to 7 days. The amounts of the Mg−Al oxides were 0.2 g and 0.25−5.0 times the stoichiometric quantities indicated by Eqs. 3 and 4:
The suspended solutions were filtered, and the Cl− and SO4 2− concentrations of the filtrates were determined using a Dionex DX-120 ion chromatograph and a Dionex model AS−12A column (eluent: 2.7 mM Na2CO3 and 0.3 mM NaHCO3; flow rate: 1.3 mL min−1). The products were identified by X-ray diffraction (XRD) analysis using Cu Kα radiation.
Results and discussion
Figure 1 shows the extent of Cl− or SO4 2− removal over time at various temperatures for a suspension of Mg−Al oxide (Mg/Al = 4) in seawater. The degree of the Cl− removal increased with time, indicating its uptake from seawater by Mg−Al oxide (Mg/Al = 4), and absorption also increased with increasing temperature. The maximum extent of Cl− removal was 67 % at 60 °C after 360 min. For SO4 2−, the percent removal increased with time at 10 and 30 °C, again reflecting the uptake of SO4 2− from seawater by Mg−Al oxide (Mg/Al = 4). The removal of SO4 2− increased as the temperature rose from 10 to 30 °C, and reached approximately 80 % at both temperatures in 360 min. However, at 60 °C, SO4 2− removal increased rapidly with time, and then decreased. This suggested a desorption of SO4 2− after uptake. Since the degree of Cl− removal at 60 °C increased with time, the desorption of SO4 2− was probably caused by anion exchange between SO4 2− in the interlayer of Mg−Al LDH and Cl− in solution. This indicates that higher temperature promotes the anion exchange, caused by higher concentration of Cl− than SO4 2−.
Figure 2 shows the XRD patterns for the Mg−Al oxide (Mg/Al = 4) and the products obtained by its suspension in seawater at 60 °C over several time periods. The XRD peaks ascribed to the Mg−Al oxide disappeared in 10 min, and peaks ascribed to hydrotalcite (JCPDS card 22-700), a naturally occurring hydroxycarbonate of magnesium and aluminum (Mg6Al2(OH)16CO3·4H2O) with a layered double hydroxide structure, increased with time. This suggested the regeneration of a Mg−Al LDH intercalated with Cl− and SO4 2− from the Mg−Al oxide. The Cl− and SO4 2− ions were thus removed from seawater by this regenerative process.
Figure 1 showed that the degree of Cl−removal is directly proportional to temperature at any given time. The accelerated rate of Cl− removal at higher temperatures implied that the reaction proceeded under chemical reaction control. Under the assumption that Cl− removal was governed by first-order kinetics, and using the results shown in Fig. 1, we determined the rate of Cl− removal between 10 and 60 °C according to Eq. 5.
where x is the degree of Cl− removal, t is the reaction time, and k (min−1) is the rate constant for Cl− removal. Figure 3 shows the first-order plots of Cl− removal by a suspension of Mg−Al oxide (Mg/Al = 4) in seawater at various temperatures. The plots showed good linearity at all temperatures, confirming the assumption that Cl− removal could be represented by a first-order reaction. The apparent rate constants at 10, 30, and 60 °C were 8.3 × 10−4, 5.8 × 10−3, and 6.7 × 10−2 min−1, respectively. Thus, the apparent rate constant clearly increased with increasing temperature. However, the removal of SO4 2− could not be represented by a first-order reaction. Therefore, SO4 2− removal was described by pseudo second-order kinetics according to Eq. 6 (Ho and McKay 1999).
where q e (mmol g−1) is the amount of SO4 2− removal at equilibrium, q t (mmol g−1) is the amount of SO4 2− removal at reaction time, t, and k (g mmol−1 min−1) is the rate constant for SO4 2− removal. Integration of Eq. 6 gives
A first-order reaction postulates a reaction which depends on the concentration of the adsorbate. A pseudo second-order reaction postulates a reaction which is strongly affected by electrostatic interactions between the adsorbent and adsorbate. That is, a pseudo second-order reaction considers the variation of SO4 2− concentration in the adsorbent and solution. Figure 4 shows pseudo second-order plots for SO4 2− removal by a suspension of Mg−Al oxide (Mg/Al = 4) in seawater at various temperatures. The plots show good linearity at all temperatures, confirming that SO4 2− removal can be represented by a pseudo second-order reaction. This result may be attributed to the large charge density of SO4 2−, which strongly interacts with Mg−Al oxide (Mg/Al = 4). The apparent rate constants at 10, 30, and 60 °C were 2.5 × 10−1, 2.2, and 2.0 × 10 g mmol−1 min−1, respectively. Thus, the apparent rate constant clearly increased with increasing temperature. Arrhenius plots of k, determined from the slopes of the lines in Figs. 3 and 4, are shown in Fig. 5; these plots yield apparent activation energies of 68.5 and 68.0 kJ mol−1 for Cl− and SO4 2−, respectively. These values confirmed that the removal of Cl− and SO4 2− by Mg−Al oxide (Mg/Al = 4) proceeded under chemical reaction control.
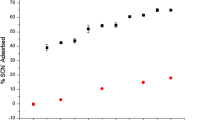
Figure 6 shows the extent of Cl− or SO4 2− removal versus time for a suspension of Mg−Al oxide (Mg/Al = 2) in seawater. Chloride removal increased with time, indicating the uptake of Cl− from seawater by the oxide. The degree of Cl− removal also increased with increasing temperature at all time points. Cl− removal reached a maximum of 34 % at 60 °C and 60 min. The degree of SO4 2− removal increased rapidly with time, also indicating the absorption of SO4 2− from seawater by Mg−Al oxide (Mg/Al = 2). The degree of SO4 2− removal increased with increasing temperature, and the maximum absorption was 99 % at 60 °C after 20 min. By comparing Figs. 1 and 6, Cl− removal by Mg−Al oxide (Mg/Al = 4) was higher than that by Mg−Al oxide (Mg/Al = 2), whereas the reverse capability was observed for SO4 2− removal. Therefore, a magnesium/aluminum ratio of four was superior for Cl− removal, and a magnesium/aluminum ratio of two was superior for SO4 2− removal.
Figure 6 shows the degree of Cl−removal directly proportional to temperature at any time. The accelerated rate of Cl− removal at higher temperatures implied that the reaction proceeded under chemical reaction control. However, Cl− removal could not be represented by a first-order reaction. Therefore, Cl− removal was described by pseudo second-order kinetics according to Eq. 6. Figure 7 displays pseudo second-order plots of Cl− removal by the suspension of Mg−Al oxide (Mg/Al = 2) in seawater at various temperatures. The plots showed good linearity at all temperatures, confirming that Cl− removal could be represented by a pseudo second-order reaction. The apparent rate constants clearly increased with increasing temperature, with values of 3.6 × 10−2, 1.5 × 10−1, and 4.8 × 10−1 g mmol−1 min−1 at 10, 30, and 60 °C, respectively. Similarly, SO4 2− removal by Mg−Al oxide (Mg/Al = 2) could not be described by a first-order reaction. Therefore, the SO4 2− removal was also represented by pseudo second-order kinetics according to Eq. 6. Pseudo second-order plots of SO4 2− removal by the suspension of Mg−Al oxide (Mg/Al = 2) in seawater at various temperatures are presented in Fig. 8. The good linearity of the plots at all temperatures confirmed the validity of the pseudo second-order representation for SO4 2− removal. The apparent rate constants clearly increased with increasing temperature, with values of 7.7 × 10−1, 3.9, and 3.7 × 10 g mmol−1 min−1 at 10, 30, and 60 °C, respectively. Arrhenius plots of k, determined from the slopes of the lines in Figs. 7 and 8, are shown in Fig. 9; these plots yield apparent activation energies of 40.2 and 60.8 kJ mol−1 for Cl− and SO4 2−, respectively. These values confirmed that the removal of Cl− and SO4 2− by Mg−Al oxide (Mg/Al = 2) proceeded under chemical reaction control.
Figure 10 shows the effect of Mg−Al oxide loading on the degree of Cl− or SO4 2− removal after the suspension of Mg−Al oxide in seawater at 30 °C for 24 h. For both Mg−Al molar ratios, the extent of Cl− and SO4 2− removal increased with increasing Mg−Al oxide quantity. For the Mg−Al oxide loadings between 0.25 and 1.25 molar equivalents, Mg−Al oxide (Mg/Al = 4) was superior to Mg−Al oxide (Mg/Al = 2) for Cl− removal, whereas the opposite was true for SO4 2− removal, as mentioned above. For Mg−Al oxide amounts above 2.0 molar equivalents, the removal of Cl− and SO4 2− for Mg−Al oxide (Mg/Al = 4) and of SO4 2− for Mg−Al oxide (Mg/Al = 2) were over 90 %. However, the Cl− removal for Mg−Al oxide (Mg/Al = 2) was only 55 % at quantity loading of 4.0 molar equivalents. The decrease in the Mg/Al molar ratio would have caused an increase in the positive charge of the host layers in the reconstructed Mg−Al LDH (Sato et al. 1987), and OH− ions, with a charge density greater than that of Cl−, would presumably have intercalated within the interlayer to compensate for the increased positive charge (Kameda et al. 2006). Figures 7 and 8 showed that Cl− and SO4 2− removal by Mg−Al oxide (Mg/Al = 2) could be represented by a pseudo second-order reaction, which was attributed to the large positive charge of the host layers for the reconstructed Mg−Al LDH (Mg/Al = 2), which strongly interacted with Cl− and SO4 2−. Furthermore, Figs. 5 and 9 showed that the apparent activation energies for Cl− and SO4 2− removal by Mg−Al oxide (Mg/Al = 2) were lower than those by Mg−Al oxide (Mg/Al = 4), which was attributed to the larger electrostatic interaction between the adsorbent and adsorbate for Mg/Al = 2 than Mg/Al = 4.
Figure 11 shows the adsorption isotherms for Cl− adsorption by the Mg−Al oxides. For both Mg/Al molar ratios, the equilibrium adsorption amount increased with increasing equilibrium concentration. The curves were considered to be Langmuir-type adsorption isotherms. This hypothesis was confirmed by arranging the experimental data according to the Langmuir equation, which can be expressed as
where q e (mmol g−1) is the equilibrium adsorption amount, C e (mmol L−1) is the equilibrium concentration, q m (mmol g−1) is the maximum adsorption amount, and K L is the equilibrium adsorption constant. Equation 8 can be converted to Eq. 9:
Figure 12 shows C e/q e versus C e plots for the adsorption isotherms of Cl− adsorbed by the Mg−Al oxides. Good linearity was obtained, indicating that this reaction can be expressed as a Langmuir-type adsorption. The reaction results from monolayer adsorption, an intercalation of Cl− in the interlayer space of the Mg−Al LDH. The values of q m and K L, determined from the slopes and intercepts of the lines in Fig. 12, were 11.7 mmol g−1 and 8.9 for Mg/Al = 4, and 7.9 mmol g−1 and 5.5 for Mg/Al = 2, respectively. The Gibbs free energy change (ΔG) for Cl− removal was estimated by the following equation:
where R is the gas constant, 8.314 J K−1 mol−1, and T (K) is absolute temperature. ΔG values were calculated to be −5.5 and −4.3 kJ mol−1 for Mg/Al = 4 and 2, respectively. Thus, the uptake of Cl− from seawater by the Mg−Al oxides proceeded spontaneously.
The adsorption isotherms for the absorption of SO4 2− by the Mg−Al oxides are shown in Fig. 13. For both Mg/Al molar ratios, the equilibrium adsorption amount increased with increasing equilibrium concentration. These curves are also considered to be Langmuir-type adsorption isotherms, which was similarly confirmed by arranging the experimental data according to the Langmuir equation. Figure 14 displays C e/q e versus C e plots for the adsorption isotherms of SO4 2− adsorbed by the Mg−Al oxides. Again, good linearity was obtained, which indicated that this reaction could be expressed as a Langmuir-type adsorption. This reaction is derived from monolayer adsorption, indicating the intercalation of SO4 2− in the interlayer space of the Mg−Al LDH. The values of q m and K L, determined from the slopes and intercepts of the lines in Fig. 14, were 1.8 mmol g−1 and 6.2 × 103 for Mg/Al = 4, and 2.0 mmol g−1 and 6.0 × 102 for Mg/Al = 2, respectively. ΔG values for SO4 2− were calculated to be −22 and −16 kJ mol−1 for Mg/Al = 4 and 2, respectively. Thus, the uptake of SO4 2− from seawater by the Mg−Al oxides also proceeded spontaneously.
Conclusion
The Mg−Al oxides simultaneously absorbed Cl− and SO4 2− from seawater, in a process that generated a Mg−Al LDH intercalated with Cl− and SO4 2−. Mg−Al oxide (Mg/Al = 4) was superior to Mg−Al oxide (Mg/Al = 2) for Cl− removal, and whereas the reverse capability was observed for SO4 2− removal. Chloride removal by Mg−Al oxide (Mg/Al = 4) could be represented by a first-order reaction, however, sulfate removal over the same substrate required a pseudo second-order reaction representation. The apparent activation energies were 68.5 and 68.0 kJ mol−1 for Cl− and SO4 2− removal by Mg−Al oxide (Mg/Al = 4), respectively, confirming that these processes proceeded under chemical reaction control. The removal of both Cl− and SO4 2− by Mg−Al oxide (Mg/Al = 2) could be represented by pseudo second-order reactions. Here, the apparent activation energies were 40.2 and 60.8 kJ mol−1 for Cl− and SO4 2−, respectively, also indicating processes under chemical reaction control. Furthermore, the adsorption isotherms for Cl− and SO4 2− adsorbed by the Mg−Al oxides could be expressed by Langmuir-type adsorptions. This reaction is derived from monolayer adsorption, indicating the intercalation of Cl− and SO4 2− in the interlayer space of the Mg−Al LDH. For Cl−, the maximum adsorption amount and equilibrium adsorption constant were 11.7 mmol g−1 and 8.9 for Mg/Al = 4, and 7.9 mmol g−1 and 5.5 for Mg/Al = 2, respectively. The Gibbs free energy changes for Cl− removal were −5.5 and −4.3 kJ mol−1 for Mg/Al = 4 and 2, respectively. The uptake of Cl− from seawater by Mg−Al oxide proceeded spontaneously. For SO4 2−, the maximum adsorption amount and equilibrium adsorption constant were 1.8 mmol g−1 and 6.2 × 103 for Mg/Al = 4, and 2.0 mmol g−1 and 6.0 × 102 for Mg/Al = 2, respectively. The Gibbs free energy changes for SO4 2− were −22 and −16 kJ mol−1 for Mg/Al = 4 and 2, respectively. The uptake of SO4 2− from seawater by Mg−Al oxide also proceeded spontaneously.
References
Allmann R (1968) The crystal structure of pyroaurite. Acta Crystallogr B 24:972–977
Chubar N (2011) New inorganic (an)ion exchangers based on Mg-Al hydrous oxides: (Alkoxide-free) sol-gel synthesis and characterization. J Colloid Interface Sci 357:198–209
Halajnia A, Oustan S, Najafi N, Khataee AR, Lakzian A (2012) The adsorption characteristics of nitrate on Mg−Fe and Mg−Al layered double hydroxides in a simulated soil solution. Appl Clay Sci 70:28–36
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Ingram L, Taylor HFW (1967) The crystal structures of sjogrenite and pyroaurite. Miner Mag 36:465–479
Kameda T, Miyano Y, Yoshioka T, Uchida M, Okuwaki A (2000) New treatment methods for waste water containing chloride ion using magnesium−aluminum oxide. Chem Lett 29:1136–1137
Kameda T, Yoshioka T, Uchida M, Miyano Y, Okuwaki A (2002) New treatment method for dilute hydrochloric acid using magnesium-aluminum oxide. Bull Chem Soc Jpn 75:595–599
Kameda T, Yabuuchi F, Yoshioka T, Uchida M, Okuwaki A (2003) New method of treating dilute mineral acids using magnesium-aluminum oxide. Wat Res 37:1545–1550
Kameda T, Yoshioka T, Hoshi T, Uchida M, Okuwaki A (2005) The removal of chloride from solutions with various cations using magnesium-aluminum oxide. Sep Purif Tech 42:25–29
Kameda T, Yoshioka T, Hoshi T, Uchida M, Okuwaki A (2006) Treatment of hydrochloric acid with magnesium-aluminum oxide at ambient temperatures. Sep Purif Tech 51:272–276
Lv T, Ma W, Xin G, Wang R, Xu J, Liu D, Liu F, Pan D (2012) Physicochemical characterization and sorption behavior of Mg-Ca-Al (NO3) hydrotalcite-like compounds toward removal of fluoride from protein solutions. J Hazard Mater 237–238:121–132
Miyata S (1983) Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner 31:305–311
Sato T, Tezuka M, Endo T, Shimada M (1987) Kinetics of anion uptake by rock salt-type magnesium aluminum oxide solid solutions. React Solids 3:287–295
Wu P, Zhang Q, Dai Y, Zhu N, Li P, Wu J, Dang Z (2011) Removal of reactive brilliant orange X-GN from aqueous solutions by Mg-Al layered double hydroxides. Clays Clay Miner 59:438–445
Yu XY, Luo T, Jia Y, Xu RX, Gao C, Zhang YX, Liu JH, Huang XJ (2012) Three-dimensional hierarchical flower-like Mg-Al-layered double hydroxides: highly efficient adsorbents for As(V) and Cr(VI) removal. Nanoscale 4:3466–3474
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kameda, T., Oba, J. & Yoshioka, T. Simultaneous removal of Cl− and SO4 2− from seawater using Mg−Al oxide: kinetics and equilibrium studies. Appl Water Sci 7, 129–136 (2017). https://doi.org/10.1007/s13201-014-0224-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-014-0224-4