Abstract
The family Lamippidae (Cyclopoida) are endosymbionts mainly occurring in shallow water octocorals and records from deep-sea corals are few. Here we investigated the relationship between the lamippid Gorgonophilus canadensis Buhl-Mortensen & Mortensen, 2004 and its host the deep-sea coral Paragorgia arborea. Twenty-one specimens of G. canadensis was found inside eight gall-like structures on a P. arborea colony collected in 2010 at 318 m depth off Norway. The galls contained on average 1.6 females, 1.0 males, and 7.5 egg sacs estimated to contain 400 eggs each. Females were larger than males (4.6 mm compared to 2.0 mm). The gall volume increased with the number of egg sacs, females, and the length of females inside, the latter correlation was significant (p < 0.05). The number of egg sacs in galls was positively correlated with the abundance and length of females (p < 0.05), and by adding Canadian data from 17 galls the relation between egg sacs and numbers of females and males in galls became stronger (p < 0.01 and p < 0.05, respectively). Scanning electron microscopy revealed that this highly modified endoparasite has thoracic appendages with non-segmented flexible spines with a specialized structure at their tips through which threads are excreted. We speculate that this adaptation could relate to feeding or attachment of egg sacs inside the galls. Thread production has rarely been reported for copepods and we explore its function in the group as well as other crustaceans. The age and size of the parasite, and the introduction to and release from the host is also discussed.
Similar content being viewed by others
1 Introduction
The endoparasitic gall-forming copepod Gorgonophilus canadensis Buhl-Mortensen & Mortensen 2004a lives inside the gorgonian coral Paragorgia arborea (Linnaeus 1758). This copepod was recorded for the first time in 2001 as part of a study on coral distribution in Atlantic Canada on the shelf and slope off Nova Scotia (Mortensen et al. 2006) and described as a new species and genus (Buhl-Mortensen and Mortensen 2004a). In 2010, it was also discovered in Norwegian waters during a MAREANO (Marine Areal Database for Norwegian Coasts and Sea Areas) seafloor mapping campaign. Here we describe the Norwegian material of this parasite to increase the understanding of the complicated and poorly understood relation between the parasite and its coral host.
G. canadensis belongs to the copepod family Lamippidae, all species of which are obligate endoparasites in octocorals (Buhl-Mortensen and Mortensen 2004a). They live inside galls formed by the coral (hollow structures of different shapes), or in the coral’s digestive cavities or polyps (Fig. 1) (Bouligand 1960, 1966). There are 54 species and 11 genera of lamippid copepods of which only five species induce formation of galls in soft corals (Williams et al. 2016; Korzhavina et al. 2021). The Lamippidae has been divided into two subfamilies (Lamippinae and Linaresiinae) that collectively contain eleven genera (Stock 1988; Williams et al. 2016; Korzhavina et al. 2021). Gorgonophilus Buhl-Mortensen & Mortensen 2004a is monotypic and is one of the 9 genera of Lamippinae.
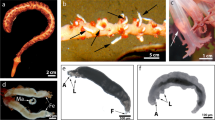
Schematic longitudinal section of octocorals with lamippid species in the coral coenosarc. a The host Alcyonium palmatum with the species: A. Lamippina aciculifera, F. Lamippella faurei, and E. Enalcyonium rubicundum. b The host Paramuricea chamaelion with the species M. Linaresia mammillifera (females and males), P. Enalcyonium parvum, and S. Enalcyonium setigerum inside. The males are in general smaller than females and are reported to move freely in the coenosarc. The highly modified and large females of L. mammillifera and Gorgonophilus canadensis probably have a permanent position within a polyp or gall. Figure modified from: Bouligand 1960
The geographic distribution of G. canadensis is linked to the host coral P. arborea which is widely distributed from the Norwegian Sea to the northwest Atlantic and it occurs mainly from 200 to 600 m in depth and at temperatures of 3–8 °C (for a review see Buhl-Mortensen et al. 2015). P. arborea acts as a host for a rich assemblage of crustacean symbionts of which G. canadensis is the only one known to change its growth form (Buhl-Mortensen and Mortensen 2004b, 2005).
The purpose of this study was to investigate the ecological association between G. canadensis and P. arborea, including new morphological details on this highly specialized copepod parasite. We describe the morphology of the galls, the position of the parasite (females, males and egg sacs) within the galls, and analyze the relationship between gall volume and the number of females, males, and egg sacs within galls. These observations are compared with data from Canada (Buhl-Mortensen and Mortensen 2004a). Scanning electron microscopy was used to examine morphological details of specialized thoracic appendages of this endoparasitic copepod and thread production by a parasitic copepod is reported for the first time.
2 Material and methods
The galls studied were all from a single colony of Paragorgia arborea that was sampled in an epibenthic sledge during a MAREANO cruise in the Norwegian Sea in 2010, station R534 (70° 41.61'N, 18o 38.58'E) at 318 m. Eight segments with galls were cut off from the coral branches and preserved in 70% ethanol. Each gall was assigned a number to keep track of content and to be able to relate content to gall morphology. The height (H) and horizontal width (W) of the galls was measured and volume was estimated using the ellipsoid formula: Volume = 4/3 * π * A * B * C where A – C are the radius for the ellipsoid in all three dimensions. A = gall height/2, B = gall width/2, and C = the smallest of A or B.
The number of “chimneys” (a protruding structure on top of the gall) was recorded, and their length measured (Fig. 2). The galls were carefully dissected to document the content and provide an overview of the inner arrangement (Fig. 3). In some galls, the positions of females were documented. Female and male parasites inside the gall were counted and measured by length. Egg sacs were counted; length of only two egg sacs were measured due to their brittleness. All dissected material was stored on 70% alcohol. For photographs and measurements, a Leica stereomicroscope connected to a digital camera and the program QImaging were used. The relation between the volume of galls, and the number of females, males, and egg sacs within galls was investigated using linear regression analysis. The analysis was conducted on the eight Norwegian galls described here, and on an increased dataset by adding 17 galls from a Canadian material (of in total 23 galls) for which single gall content was available from Buhl-Mortensen and Mortensen (2004a).
a Branch of the deep-water coral Paragorgia arborea (adopted from: Buhl-Mortensen and Mortensen 2005) scale bar 5 cm. b a gall, with three chimneys from the outer parts of a coral branch and with several normal polyps below, scale bar 1 cm
Large and intact female and male individuals were selected for detailed morphological studies using Scanning Electron Microscopy (SEM). For SEM preparation, specimens were dehydrated in an ascending ethanol series, starting with 70% and ending with 100% (10 min per dilution through 95% and 15 min × 3 for 100%). Drying of specimens was completed with a Samdri 795 Critical Point Dryer and then specimens were mounted on aluminum stubs followed by coating with gold using an EMS-550 sputter coater. Specimens prepared for SEM were examined using a FEI Quanta 250 SEM. Figures were produced using Adobe Photoshop and measurements of structures were made using ImageJ software.
3 Results
The galls mainly occurred on the outer parts of the branches of the host colony. On top of each gall one to three chimney-like structures were present, representing a possible pathway for passage of larvae from the inside of the gall to the surroundings (Figs. 2, 3 and 4, Table 1). The male, female, and egg sacs were often situated close to the inner wall of the gall, and the large egg sacs that were attached to the wall were easily destroyed during dissection (Figs. 3 and 4). Females were often oriented head down in the gall, away from the upper parts with the chimneys.
Morphology
In general, the female morphology matches the original species description with a spindle-shaped body with weak segmentation and the cephalothorax was bent downwards, often at a steeper angle in females than males (Fig. 5a). The females have large lateral lobes on each side of the head that is typical for this species and were 4–5.7 mm in length. The males are 1.9–2.3 mm long and in general less than half of the female length. The gonopores are clearly visible on the lower part of the female body (Fig. 5b). There are two pairs of antennae; antennules appear unsegmented with setae, antennae have 3 segments. Two pairs of legs are present, both biramous with single segmented exopodite and two segmented endopodite. Exopodite and endopodites terminate with palmately arranged digitations on distal segments. In large females, the exopodites have at least eight modified spines and the endopodites have six (Fig. 4F in Buhl-Mortensen and Mortensen 2004a). The two egg sacs measured were 2.4 and 2.8 mm in length (Fig. 3) and approximately half the length of females. The egg sacs (Fig. 5d) contained eggs approximately 125 µm in diameter that are arranged in a spiral pattern within the egg sac, and a single egg sac is estimated to contain 400–500 eggs. No free larvae were encountered inside the galls but a few fully developed nauplius larvae (125 µm) were recorded among the eggs (Fig. 5c).
Photos of Gorgonophilus canadensis: a Mature female (5.2 mm) and male (1.8 mm) in lateral view. The female shows the typical forward bent posture with head and mouth close to the thoracic appendages and weak segmentation is visible. b SEM photos of mature female, ventral side with a bundle of threads visible below the head and gonopores on the abdomen. c Photo of nauplius (length = 125 μm) from egg in egg sac, d SEM of an egg sac showing spiral packing of eggs, e SEM detail of the female gonopore
Gall size and content
Size and estimated volume of galls varied from the smallest with a height and width of 3.1 and 5.2 mm, respectively, and an estimated volume of 0.209 cm3 to the largest with the corresponding measures being 12.5 and 8.2 mm and 3.52 cm3 (Table 1). On average, galls contained 1.6 females, 1.0 males and 7.3 egg sacs. The two largest galls both housed 3 females and 1 male together with 10 and 17 egg sacs; in contrast, only a single male individual inhabited the smallest gall (Table 1). Numbers of females, length of females, and number of egg sacs increased with increased estimated gall volume (Fig. 6a−e). The relation between gall volume and length of females was significant (r = 0.53; p < 0.05, df 12). The number of egg sacs in the galls showed a positive and significant correlation with number and length of females, r = 0.84 (p < 0.05, df 7) and r = 0.56 (p < 0.05, df 12), respectively (Fig. 6d, e). Comparing our Norwegian results with data from Canada published by Buhl-Mortensen and Mortensen (2004a) (Table 1) showed that the Canadian material had a larger range in numbers of males and females per gall with a maximum of five and seven, respectively. The largest female in the Canadian material was 8.5 mm while in the Norwegian material it was 5.7 mm. We also analyzed the larger combined dataset of 25 that included eight Norwegian galls and 17 galls from the Canadian material (Fig. 7). The correlation between numbers of females and egg sacs in galls (Fig. 7a, b) was positive and significant (r = 0.60, p < 0.01) when one outlier of 7 females in one gall was excluded. The relation between presence of males and egg sacs in galls (Fig. 7c) was also significant (r = 0.40, p < 0.05).
The relation betweeen estimated gall volume and content of; a number of females, b length of females, and c number of egg sacs. The relation berween number of egg sacs in a gall and d number of females and e length of females in the gall. Based on the Norwegian material of eight galls containing 13 females. *: p < 0.05
Plots of the relation between gall content of egg sacs, females and males based on the 25 galls, 8 galls from the new Norwegian data and 17 galls from the Canadian data reported by Buhl-Mortensen & Mortensen (2004a). a and b relation between number of females and egg sacs, in b the extreme value of 7 females in one gall is excluded. c numbers of males in relation to egg sacs. **: p < 0.01, *: p < 0.05
New observation of highly specialized appendages
SEM analysis of the new Norwegian material shows that the spines of the legs are tubular and 50–100 µm long (Fig. 8b); the distal end of the spines have a ring of approximately 30 rounded digitations surrounding an opening (Fig. 8c, d). The spines appear elastic, flexible, and have no segmentation (Fig. 8b). In some specimens a bundle of threads appeared to be excreted from this distal opening, forming a dense matt of threads below the female head (Figs. 8c, 9a, b). The threads appear to be excreted from glands opening below the rounded digitations at the tips of the spines. High magnification of the excreted threads reveal that they are approximately 1–3 µm in diameter and exhibit round, droplet-like formations along the lenth (Fig. 9c, d).
SEM photos of Gorgonophilus canadensis: a Female head with antennae and upper body with the two pairs of thoracic appendages. b Close up of appendages below the oral cone. c Lateral view of the tip of a single spine on a thoracic appendage with multiple threads being excreted. d Distal end of a spine showing rounded digitations at the tips
4 Discussion
The content of galls (Table 1) was similar to observations in the Canadian study of the parasite, where the average numbers in galls was 2.0 females, 1.2 males and 7.5 egg sacs (Buhl-Mortensen and Mortensen 2004a). However, the size of the female and male described in the Canadian material (8.5 mm and 2.5 mm, respectively), and the maximum number of males and females in a gall, were considerably larger than in the Norwegian material. This was probably because the Canadian material included three times more galls than the Norwegian material and came from four different colonies, while the Norwegian material was from one colony. However, the Norwegian material was in better condition, stored directly in 70% ethanol after collecting, while the Canadian galls were from frozen colonies, affecting the quality of their content.
Size, age, and content of galls
The galls with the largest volume contained females and egg sacs above average, and the smallest gall had no egg sacs and was inhabited by the smallest female recorded and a small male (Table 1). Gall volume showed a positive correlation with abundance of parasites and egg sacs inside (Fig. 6). It is not known how the copepods induce gall formation or how it interacts with its host to cause the gall to increase in size as the inside population increases (but see review of gall-inducing lamippids by Korzhavina et al. 2021). However, the large numbers of females/gall (e.g., 7 in a single gall) could indicate that the Canadian galls were older and had grown to a substantial size; unfortunately, the galls in this material were not measured.
We have shown that the galls of G. canadensis not only house the large females and the minute males, but the walls are also used to anchor the egg sacs. This may explain the close relationship between gall size and number of egg sacs and females. Based on the linear relation between number of females (NF) and gall volume (GV) from the Norwegian material: NF = 0.0006 × GV(mm3) + 0.45 (see Fig. 6a) a gall with 7 females would be predicted to have a volume of 10917 (mm3). The female length to gall volume relation from the Norwegian specimens: FL(mm) = 0,0003 × GV(mm3) + 4.2 (see Fig. 6b) indicates that the largest female in the Canadian material may have come from a gall of approximately 14333 mm3. This suggests that the largest Canadian gall was 3–4 times larger than the largest gall in the Norwegian material (3600 mm3). The occurrence of large numbers of egg sacs together with several adult females inside a single gall of G. canadensis could indicate that the gall and parasite are long-lived and/or has multiple generation cycles inside a gall. Furthermore, we did not observe any remnants of former inhabited galls; however, at present, we can only speculate on how long a gall exist and the age of its inhabitants. Grygier (1981) similarly concluded that the gall forming ascothoracid barnacle Gorgonolaureus muzikae was long-lived based on the findings of many adult females parasitic in a Hawaiian gorgonian.
During the dissection of the eight Norwegian galls, including their chimneys, no free nauplius larvae were found and we do not know how it enters the host. Our new record of G. canadensis from Norway shows that it occurs on both sides of the North Atlantic. The intensity of parasites in hosts is often high with as many as 8 galls in a single colony, each of which includes several copepod specimens. This shows that this newly described species is widely distributed, and its larva is able to disperse well. The galls are mainly found on the outer parts of the host colony branches. We speculate that the copepod larva settles on the colony surface and enters the coenenchyma via the polyp; once inside the host, it can develop and gradually induce gall formation. The galls do not appear to have additional openings other than their chimney(s) and it has been suggested that larvae hatched in the gall could escape by using the narrow passage through the chimney (Buhl-Mortensen and Mortensen 2004a). Penney et al. (2021) suggested nauplii (~ N5 stage) of L. bouligandi were released from the host for dispersal to nearby or distant sea pen hosts. Once a host coral is infested, studies shows that lamippid copepods can move inside the host coenosarc (Bouligand 1960, and Fig. 1) and it is potentially able to parasitize other parts of the host.
In addition to G. canadensis there are four gall forming species in the lamippid family. Isidicola antarctica (Gravier 1914a, b) and Sphaerippe caligicola (Grygier 1980), appear to induce gall formations in their hosts Primnoisis formosa Gravier, 1913 and Callogorgia sp., respectively (Williams et al. 2016). According to Gravier (1914a, b), the galls inhabited by I. antarctica normally contain one small male, one small female, several juveniles and free eggs. He suggested that the parasites can orient in the gall using their antenna to detect stimuli. There might be several sensors located on their body helping them to orient inside their gall as suggested by Bouligand (1960, 1996). Linaresia magna Grygier, 1980 is a gall forming species from the Hawaiian gorgonian Placogorgia sp.. Grygier (1980) found that galls typically contained a female and male pair (rarely 2–3 males) and eggs; in some cases, females were found associated with galls produced by the ascothoracid barnacle G. muzikae and solitary males were found occasionally outside of galls (Grygier 1980). A recently discovered lamippid copepod Ptilosarcoma athyrmata Williams et al. 2016 infests leaves of the sea pen Ptilosarcus gurneyi (Gray 1860), living in the Strait of Georgia, British Columbia, Canada (Williams et al. 2016). The galls are spherical structures along the leaves, showing differences in size and shape. Females and males have an average size of 2.1 mm and 1.4 mm, respectively, and every gall contains one of each sex. G. canadensis differs both by containing an average of two females and one male per gall and by having a substantially larger size, with average measures of 4.9 mm and 2.0 mm, respectively (Table 1). Another difference is the presence of egg sacs inside the galls of P. arborea, whereas eggs were reported as loose inside the galls for all the other species.
New morphological observations
Details not reported by Buhl-Mortensen and Mortensen (2004a), include high magnification SEM photos of thoracic appendages and the position of the female gonopore. The appendages appear to be flexible and the elasticity of the cuticle allows movement without segmentation. The spines of the thoracic appendages appear to have glands on their tips through which thin threads are excreted. Threads or mucous secretions like this have only been reported in a few cases among crustaceans. A small number of copepod species are known to produce mucus to aid in food capture (Cahoon 1982; Hicks and Grahame 1979; von Vaupel Klein and Koomen 1994) or for tube-building (e.g., Chandler and Fleeger 1984; Williams-Howze et al. 1987). Similarly, the production of “silk” to build tubes has been documented in amphipods (Kronenberger et al. 2012a, b; Neretin 2016; Neretin et al. 2017) and tanaids (Kakui and Hiruta 2014, 2017; Kaji et al. 2016; Kakui et al. 2021).
The function and chemical composition of the threads produced by G. canadensis remain unknown but could be composed of mucus or a mixture of mucopolysaccharides and protein. Based on the morphology of the threads, they appear to be similar to the silk threads produced by amphipods (e.g., compare Fig. 9C herein to Fig. 3E in Neretin et al. 2017). Whether they are true spun “silk” (Vollrath and Porter 2009) is in need of further research, and future work could also include silk proteomic studies as has been done in tanaids (Kakui et al. 2021). As to the function, the excreted threads could be involved in feeding (see below) or involved in gall formation or fastening of eggs sacs within the host.
Some lamippid copepods are morphologically adapted to move around inside their host, as was described by Bouligand (1960, 1966) (see Fig. 1). Bouligand (1960) reported that mobile species have small, slender bodies (males smaller than females) and can move freely in the coenosarc. He suggest that species with the highly modified and larger females have a permanent position within a polyp or gall. The larger and highly modified females of G. canadensis are likely not able to move outside the gall while the smaller males and larvae might be able to use channels in their hosts coenosarc or move to the surface of the host and parasitize other parts of the colony. The coral genus Paragorgia is unique by having three large channels in its medulla (Sánchez 2005).
Mature females residing in the galls likely survive by eating the tissue of their host, by scraping it from the wall, as suggested by Patton (1976), Mortensen and Buhl-Mortensen (2004a) and Penney et al. (2021). The feeding biology of lamippids is poorly known, but Bouligand (1960) showed Enalcyonium feed on eggs and endodermal material from the host. G. canadensis has not been documented to be an egg predator of the host, and eggs of the host were not observed in the galls. It should be investigated whether the excreted threads could be involved in the feeding by copepods on host endodermal and/or gonadal tissue.
Bouligand (1960, 1996) examined adaptations of lamippids and noted their elastic cuticula that allowed for peristatic movement and excretion of droplets from its surface. According to his observations using light microscopy, several lamippids have “ …. at the tip of setae, a bundle of needles (or aciculae), with droplets slowly running along them…”, in some species this occurs only on the furca and others they are found on all appendages. Our observations of droplet-like formations on the threads excreted by the appendages of G. canadensis are likely not homologous with the droplets described by Hipeau-Jacquotte (1986) and Bouligand (1996). Many questions remain to be answered the functions of these features and to better understand the natural history of lamippid copepods. We hope that our study of the Norwegian material of G. canadensis will increase the understanding of the ecology of this highly specialised parasite and its relation to its coral host.
References
Bouligand Y (1960) Notes sur la famille des Lamippidae. Premier Partie Crustaceana 1:258–278
Bouligand Y (1966) Recherches récentes sur les Copépodes associés aux Anthozoaires, The Cnidarian and their Evolution. Symp Zool Soc 16:267–306
Bouligand Y (1996) Morphological singularities and macroevolution. Systematic Biology as an Historical Sciensce, Memorie della Società Italiana di Sienze Naturali e del Museo Civico di Storia Naturale di Milano, Vol. XXVII-Fascicolo I
Buhl-Mortensen L, Mortensen PB (2004a) Gorgonophilus canadensis n. gen., n. sp. (Copepoda: Lamippidae), a gall forming endoparasite in the Octocoral Paragorgia arborea ( L., 1758) from the Northwest Atlantic. Symbiosis 37:1–14
Buhl-Mortensen L, Mortensen PB (2004b) Crustacean fauna associated with the deep-water corals Paragorgia arborea and Primnoa resedaeformis. J Nat His 38:1233–1247
Buhl-Mortensen L, Mortensen PB (2005) Distribution and diversity of species Associated with Deep-sea gorgonian corals off Atlantic Canada. In: Freiwald A, Roberts JM (eds) Cold-water Corals and Ecosystems. Springer-Verlag, Berlin Heidelberg, pp 849–879
Buhl-Mortensen L, Olafsdottir SH, Buhl-Mortensen P, Burgos JM, Ragnarsson SA (2015) Distribution of nine cold-water coral species (Scleractinia and Gorgonacea) in the cold temperate North Atlantic in light of bathymetry and hydrography. Hydrobiologia 759:39–61
Cahoon LB (1982) The use of mucus in feeding by the copepod Euchirella venusta Giesbrecht. Crustaceana 43:202–204
Chandler GT, Fleeger JW (1984) Tube-building by a marine meiobenthic harpacticoid copepod. Mar Biol 82:15–19
Gravier C (1913) Seconde expédition antarctique Française (1908–1910). Alcyonaires (1re note préliminaire). Bulletin de la Museum Nationale d'Histoire naturelle Paris. 19:451–455
Gravier Ch (1914a) Isidicola atarctica, Crustacé parasite de quelques Isidae de l’Antartique sud-américaine. Deuxième Expédition Antartique Francaise (1908–1910)’, Les Alcyonaires, Appendice, pp. 99–110
Gravier Ch (1914b) Sur un type nouveau de Crustacé parasite d’Alcyonaires de l’Antartique sud-américaine. CR Acad Sci Paris 158:354–356
Gray JE (1860) II. Revision of the family Pennatulidae, with descriptions of some new species in the British Museum. Annals and Magazine of Natural History: Zoology, Botany and Geology, Series 3. 5(25–30):20–25
Grygier MJ (1980) Two new lamippid copepods parasitic on gorgonians from Hawaii in the Bahamas. Proc Biol Soc Wash 93(3):662–673
Grygier MJ (1981) Gorgonolaureus muzikae sp. Nov. (Crustacea: Ascothoracida) parasitic on a hawaiian gorgonian, with special reference to its protandric hermaphroditism. J Nat His 15(6):1019–1045
Hicks GR, Grahame J (1979) Mucus production and its role in the feeding behaviour of Diarthrodes nobilis (Copepoda: Harpacticoida). J Mar Biol Assoc UK 59:321–330
Hipeau-Jacquotte R (1986) A new cephalic type of presumed sense organ with naked dendritic ends in the atypical male of the parasitic copepod Pachypygus gibber (Crustacea). Cell Tiss Res 245:29–35
Kaji T, Kakui K, Miyazaki N, Murata K, Palmer AR (2016) Mesoscale morphology at nanoscale resolution: serial block-face scanning electron microscopy reveals fine 3D detail of a novel silk spinneret system in a tube-building tanaid crustacean. Front Zool 13:1–9
Kakui K, Hiruta C (2014) Diverse pereopodal secretory systems implicated in thread production in an apseudomorph tanaidacean crustacean. J Morph 275:1041–1052
Kakui K, Hiruta C (2017) Tube construction by a tanaidacean crustacean using a novel mucus secretion system involving the anal opening. Zool Lett 3:1–7
Kakui K, Fleming JF, Mori M, Fujiwara Y, Arakawa K (2021) Comprehensive transcriptome sequencing of Tanaidacea with proteomic evidenes for their silk. Genome Biol Evol 13:evab281. https://doi.org/10.1093/gbe/evab281
Korzhavina OA, Reimer JD, Ehrlich H, Ivanenko VN (2021) Global diversity and distribution of Lamippidae copepods symbiotic on Octocorallia. Symbiosis 83:265–277. https://doi.org/10.1007/s13199-021-00750-y
Kronenberger K, Dicko C, Vollrath F (2012a) A novel marine silk. Naturwissenschaften 99:3–10
Kronenberger K, Moore PG, Halcrow K, Vollrath F (2012b) Spinning a marine silk for the purpose of tube-building. J Crust Biol 32:191–202
Linnaeus C (1758) Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio decima, reformata [10th revised edition] 1:824
Mortensen PB, Buhl-Mortensen L, Gordon Jr. DC (2006) Distribution of deep-water corals in Atlantic Canada. Proceedings of the 10th International Coral Reef Symposium, Okinawa. pp 1832–1848
Neretin NY (2016) The morphology and ultrastructure of “amphipod silk” glands in Ampithoe rubricata (Crustacea, Amphipoda, Ampithoidae). Biol Bull 43:628–642
Neretin NY, Zhadan AE, Tzetlin AB (2017) Aspects of mast building and the fine structure of “amphipod silk” glands in Dyopedos bispinis (Amphipoda, Dulichiidae). Contrib Zool 86:145–168
Patton WK (1976) Animal associates of living reef corals. In: Jones OJ, Endean R (eds) Biology and geology of coral reefs, vol III. Academic Press, New York, pp 1–39
Penney HD, Baillon S, Hamel JF, Pête J, Mercier A (2021) Morphology and biology of the endoparasitic copepod Lamippe bouligandi from the bathyal sea pen Anthoptilum grandiflorum. Symbiosis 85:233–248
Sánchez JA (2005) Systematics of the bubblegum corals (Cnidaria: Octocorallia: Paragorgiidae) with description of new species from New Zealand and Eastern Pacific. Zootaxa 1014:1–72
Stock JH (1988) Lamippidae (Copepoda: Siphonostomatidae) parasitic in Alcyonium. J Mar Biol Assoc UK 68:351–359
von Vaupel Klein JC, Koomen P (1994) The possible origin of mucus jets used for immobilizing prey in species of Euchirella (Copepoda, Calanoida, Aetideidae). I. Theoretical considerations in relation to swimming and feeding behaviour. Crustaceana 66(2):184–204
Vollrath F, Porter D (2009) Silks as ancient models for modern polymers. Polymer 50(24):5623–5632
Williams JD, Anchaluisa B, Boyko CB, McDaniel N (2016) Description of a new endoparasitic copepod genus and species (Lamippidae) that induces gall formation in leaves of the sea pen Ptilosarcus gurneyi (Octocorallia) from British Columbia. Mar Biodivers 48:1325–1335
Williams-Howze J, Silverman H, Fleeger JW (1987) Internal morphology related to tube-building in the meiobenthic copepod Pseudostenhelia wellsi. J Crust Biol 7(1):171–181
Acknowledgements
This study was conducted as a part of a course at the University of Bergen in cooperation with the Institute of Marine Research (IMR) and the Mareano program. We are grateful to Pål Buhl-Mortensen (Institute of Marine Research, Bergen) for preserving and providing the samples. Special thanks are due to Arne Hassel for providing all necessary laboratory space, equipment, and guidance for conducting this research. The comments of an anonymous reviewer are appreciated and improved the manuscript. This research was also supported, in part, by a grant to J. D. Williams from the National Science Foundation (DBI-1337525).
Funding
Open Access funding provided by Institute Of Marine Research
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buhl-Mortensen, L., Neuhaus, J. & Williams, J.D. Gorgonophilus canadensis (Copepoda: Lamippidae) a parasite in the octocoral Paragorgia arborea – relation to host, reproduction, and morphology. Symbiosis 87, 189–199 (2022). https://doi.org/10.1007/s13199-022-00866-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-022-00866-9