Abstract
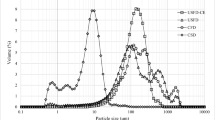
Transglutaminase (TG), which is an important enzyme for food processing, can enhance the firmness, viscosity and water binding capacity of food products by catalyzing the cross-linking reaction of proteins. Since preservation of the enzyme activity is essential, the production of microencapsulated powder form of TG can be a great challenge to maintain its initial activity. In this study, TG was microencapsulated using a freeze drying technique and the effects of homogenization conditions and coating material ratios on the enzyme activity were investigated using D-optimal combined design. Mannitol, gum arabic and casein were chosen as coating materials and different homogenization times (1–5 min) and homogenization rates (11,200–20,000 rpm) were applied. The optimum conditions which ensure the maximum enzyme activity have been determined as 11,200 rpm of homogenization rate, 1.27 min of homogenization time, and in addition a mixture of mannitol, gum arabic and casein with ratios 38.2, 40.2, and 21.6%, respectively. Most of the activity loss occurred in the homogenization stage and the coating materials preserved enzyme activity during freeze drying. At the optimum point, the remaining activity of the microencapsulated TG was 93% while that of the crude (without coating materials) TG was 64% at the same drying conditions. Moreover, the effects of the microencapsulation conditions on the physical properties of powder such as moisture content, color, particle, bulk and tapped densities, porosity and flowability were determined.


Similar content being viewed by others
References
Adamiec J, Kaminski W, Markowski AS, Strumiłło C (2006) Drying of biotechnological products. In: Mujumdar AS (ed) Handbook of industrial drying. Taylor & Francis Group, LLC, Routledge, pp 905–926
Amid M, Manap Y, Zohdi NK (2014) Microencapsulation of purified amylase enzyme from pitaya (Hylocereus polyrhizus) peel in arabic gum-chitosan using freeze drying. Molecules 19(3):3731–3743
Andrea T, Marcela F, Lucía C, Esther F, Elena M, Simona M (2016) Microencapsulation of lipase and savinase enzymes by spray drying using arabic gum as wall material. J Encapsulation Adsorpt Sci 6(04):161
Anjani K, Kailasapathy K, Phillips M (2007) Microencapsulation of enzymes for potential application in acceleration of cheese ripening. Int Dairy J 17(1):79–86
Arakawa T, Prestrelski SJ, Kenney WC, Carpenter JF (2001) Factors affecting short-term and long-term stabilities of proteins. Adv Drug Deliv Rev 46(1):307–326
Bourneow C, Benjakul S, H-Kittikun A (2012) Impact of some additives on the stability of microbial transglutaminase from Providencia sp. C1112. Asian J Food Agric 5(3):226–233
Carr RL (1965) Evaluating flow properties of solids. Chem Eng 72:163–168
Cui L, Du G, Zhang D, Liu H, Chen J (2007) Purification and characterization of transglutaminase from a newly isolated Streptomyces hygroscopicus. Food Chem 105(2):612–618
Debulis K, Klibanov AM (1993) Dramatic enhancement of enzymatic activity in organic solvents by lyoprotectants. Biotechnol Bioeng 41(5):566–571
Desai KGH, Park HJ (2005) Recent developments in microencapsulation of food ingredients. Dry Technol 23(7):1361–1394
do Vale Morais AR, do Nascimento Alencar É, Júnior FHX, de Oliveira CM, Marcelino HR, Barratt G, Elaissari A (2016) Freeze-drying of emulsified systems: a review. Int J Pharm 503(1):102–114
Fitzpatrick JJ (2005) Food powder flowability. In: Onwulata C (ed) Encapsulated and powdered foods. Taylor & Francis Group, LLC, Routledge, pp 247–260
Gaspar ALC, Góes-Favoni SPD (2015) Action of microbial transglutaminase (MTGase) in the modification of food proteins: a review. Food Chem 171:315–322
Ghadge RS, Patwardhan AW, Sawant SB, Joshi JB (2005) Effect of flow pattern on cellulase deactivation in stirred tank bioreactors. Chem Eng Sci 60:1067–1083
Gharibzahedi SMT, Yousefi S, Chronakis IS (2019) Microbial transglutaminase in noodle and pasta processing. Crit Rev Food Sci 59(2):313–327
Gharsallaoui A (2007) Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Res Int 40(9):1107–1121
Glab TK, Boratyński J (2017) Potential of casein as a carrier for biologically active agents. Top Curr Chem 375(4):71–91
Hausner HH (1967) Friction conditions in a mass of metal powder. Powder Metall 13:7–13
Heidebach T, Först P, Kulozik U (2010) Influence of casein-based microencapsulation on freeze-drying and storage of probiotic cells. J Food Eng 98(3):309–316
Izutsu KI, Yoshioka S, Terao T (1994) Effect of mannitol crystallinity on the stabilization of enzymes during freeze-drying. Chem Pharm Bull 42(1):5–8
Jinapong N, Suphantharika M, Jamnong P (2008) Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J Food Eng 84(2):194–205
Katayama K, Chin KB, Yoshihara S, Muguruma M (2006) Microbial transglutaminase improves the property of meat protein and sausage texture manufactured with low-quality pork loins. Asian Aust J Anim 19(1):102
Kawai K, Suzuki T (2007) Stabilizing effect of four types of disaccharide on the enzymatic activity of freeze-dried lactate dehydrogenase: step by step evaluation from freezing to storage. Pharm Res 24(10):1883–1890
Kieliszek M, Misiewicz A (2014) Microbial transglutaminase and its application in the food industry: a review. Folia Microbiol 59(3):241–250
Kneifel W, Seiler A (1993) Water-holding properties of milk protein products—a review. Food Struct 12(3):3
Knorr D (1998) Technology aspects related to microorganisms in functional foods. Trends Food Sci Technol 9:295–306
Koç B, Eren I, Kaymak-Ertekin F (2008) Modelling bulk density, porosity and shrinkage of quince during drying: the effect of drying method. J Food Eng 85(3):340–349
Koç B, Sakin-Yılmazer M, Kaymak-Ertekin F, Balkır P (2014) Physical properties of yoghurt powder produced by spray drying. J Food Sci Technol 51(7):1377–1383
Kuraishi C, Yamazaki K, Susa Y (2001) Transglutaminase: its utilization in the food industry. Food Rev Int 17(2):221–246
Lin YS, Chao ML, Liu CH, Tseng M, Chu WS (2006) Cloning of the gene coding for transglutaminase from Streptomyces platensis and its expression in Streptomyces lividans. Process Biochem 41(3):519–524
Liu H, Nakagawa K, Kato DI, Chaudhary D, Tadé MO (2011) Enzyme encapsulation in freeze-dried bionanocomposites prepared from chitosan and xanthan gum blend. Mater Chem Phys 129(1):488–494
Macedo JA, Sette LD, Sato HH (2011) Purification and characterization of a new transglutaminase from Streptomyces sp. isolated in Brazilian soil. J Food Biochem 35(4):1361–1372
Motoki M, Seguro K (1998) Transglutaminase and its use for food processing. Trends Food Sci Technol 9(5):204–210
Ramos A, Raven N, Sharp RJ, Bartolucci S, Rossi M, Cannio R, Lebbing J, Oost JVD, de Vos WM, Santos H (1997) Stabilization of enzymes against thermal stress and freeze-drying by mannosylglycerate. Appl Environ Microb 63(10):4020–4025
Ray S, Raychaudhuri U, Chakraborty R (2016) An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci 13:76–83
Shiga H, Joreau H, Neoh TL, Furuta T, Yoshii H (2014) Encapsulation of alcohol dehydrogenase in mannitol by spray drying. Pharmaceutics 6(1):185–194
Sun-Waterhouse D, Wadhwa SS, Waterhouse GIN (2013) Spray-drying microencapsulation of polyphenol bioactives: a comparative study using different natural fibre polymers as encapsulants. Food Bioprocess Technol 6(9):2376–2388
Taguchi S, Arakawa K, Yokoyama K, Takehana S, Takagi H, Momose H (2002) Overexpression and purification of microbial pro-transglutaminase from Streptomyces cinnamoneum and in vitro processing by Streptomyces albogriseolus proteases. J Biosci Bioeng 94(5):478–481
Thomas CR, Geer D (2011) Effects of shear on proteins in solution. Biotechnol Lett 33(3):443–456
Vikartovska A, Bucko M, Mislovicova D, Patoprsty V, Lacık I, Gemeiner P (2007) Improvement of the stability of glucose oxidase via encapsulation in sodium alginate–cellulose sulfate–poly(methylene-co-guanidine) capsules. Enzyme Microb Technol 41:748–755
Vinceković M, Viskić M, Jurić S, Giacometti J, Kovačević DB, Putnik P, Jambrak AR (2017) Innovative technologies for encapsulation of Mediterranean plants extracts. Trends Food Sci Technol 69:1–12
Yokoyama K, Nio N, Kikuchi Y (2004) Properties and applications of microbial transglutaminase. Appl Microbiol Biotechnol 64(4):447–454
Zhang Y, Zhong Q (2018) Freeze-dried capsules prepared from emulsions with encapsulated lactase as a potential delivery system to control lactose hydrolysis in milk. Food Chem 241:397–402
Zhu Y, Rinzema A, Tramper J, Bruin ED, Bol J (1998) Fed-batch fermentation dealing with nitrogen limitation in microbial transglutaminase production by Streptoverticillium mobaraense. Appl Microbiol Biotechnol 49:251–257
Acknowledgements
The authors acknowledge the Scientific and Technological Research Council of Turkey (TUBITAK, Project No: 115O216) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Isleroglu, H., Turker, I., Koc, B. et al. Optimization of microencapsulation conditions of transglutaminase by freeze drying. J Food Sci Technol 56, 4925–4937 (2019). https://doi.org/10.1007/s13197-019-03962-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03962-9