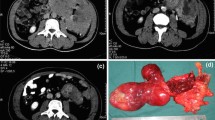
Abstract
Surgical resection with negative margins of non-metastatic gastric GISTs is considered the main therapeutic option in GISTs treatment. Neoadjuvant therapy with imatinib is associated with higher response rates in advanced GISTs. We reported 34 patients with non-metastatic gastric GISTs who underwent partial gastrectomy at the Oncology Center, Mansoura University, Egypt, after receiving a daily dose of 400 mg of imatinib as a neoadjuvant treatment in the period between October 2012 and January 2021. Twenty-two cases underwent open partial gastrectomy, and twelve cases had a laparoscopic partial gastrectomy. The median tumor size at diagnosis was 13.5 cm (range 9–26 cm) and the duration of neoadjuvant therapy was 10.91 months (range 4–12 months). Thirty-three patients had a partial response, while one patient showed progression of the disease on neoadjuvant treatment. Adjuvant therapy was conducted in 29 (85.3%) cases. Complications of neoadjuvant treatment were reported in seven cases in the form of gastritis, bleeding per rectum, fatigue, thrombocytopenia, neutropenia, and edema lower limbs. The disease-free survival (DFS) in this study was 34.53 months, and the overall survival (OS) was 37 months. Recurrence developed in two cases, gastric and peritoneal recurrence (25 and 48 months from the initial diagnosis, respectively). We have concluded that neoadjuvant treatment with imatinib for non-metastatic gastric GISTs is a safe and effective method for tumor downsizing and devitalization to allow minimally invasive and/or organ sparing surgery. Moreover, it decreases the risk of intraoperative tumor rupture and relapse, thus improving the oncological outcome of such tumors.

Similar content being viewed by others
Data Availability
The datasets generated and analyzed during the current study are not publicly available and are available from the corresponding author on reasonable request.
References
Ressing M, Wardelmann E, Hohenberger P, Jakob J, Kasper B, Emrich K, Zeissig SR (2018) Strengthening health data on a rare and heterogeneous disease: sarcoma incidence and histological subtypes in Germany. BMC Public Health 18(1):1–11. https://doi.org/10.1186/s12889-018-5131-4
Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK (2016) The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 19(1):3–14. https://doi.org/10.1007/s10120-015-0526-8
Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kitamura Y (1998) Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279(5350):577–580. https://doi.org/10.1126/science.279.5350.577
Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Demetri GD (2001) Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 344(14):1052–1056. https://doi.org/10.1056/NEJM200104053441404
Von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Ganjoo KN, Scavone JL (2018) Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 16(5):536–563. https://doi.org/10.6004/jnccn.2018.0025
Casali PG, Abecassis N, Bauer S, Biagini R, Bielack S, Bonvalot S, Blay JY (2018) Soft tissue and visceral sarcomas: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol 29:iv51–iv67. https://doi.org/10.1093/annonc/mdy096
DeMatteo RP, Gold JS, Saran L, Gönen M, Liau KH, Maki RG, Antonescu CR (2008) Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer: Interdiscip Int J Am Cancer Soc 112(3):608–615
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92(3):205–216. https://doi.org/10.1093/jnci/92.3.205
Jakob J, Hohenberger P (2018) Neoadjuvant therapy to downstage the extent of resection of gastrointestinal stromal tumors. Visc Med 34(5):359–365. https://doi.org/10.1159/000493405
Jakob J, Mussi C, Ronellenfitsch U, Wardelmann E, Negri T, Gronchi A, Hohenberger P (2013) Gastrointestinal stromal tumor of the rectum: results of surgical and multimodality therapy in the era of imatinib. Ann Surg Oncol 20(2):586–592. https://doi.org/10.1245/s10434-012-2647-1
Wilkinson MJ, Fitzgerald JEF, Strauss DC, Hayes AJ, Thomas JM, Messiou C, Judson I (2015) Surgical treatment of gastrointestinal stromal tumor of the rectum in the era of imatinib. Br J Surg 102(8):965–971. https://doi.org/10.1002/bjs.9818
Stiekema J, Kol S, Cats A, Yazdi AT, van Coevorden F, van Sandick JW (2015) Surgical treatment of gastrointestinal stromal tumors located in the stomach in the imatinib era. Am J Clin Oncol 38(5):502–507. https://doi.org/10.1097/COC.0b013e3182a78de9
C Yoo, MH Ryu, BW Kang, SK Yoon, BY Ryoo, HM Chang, YK Kang (2010). A cross-sectional study of imatinib plasma trough levels in patients with advanced gastrointestinal stromal tumors: impact of gastrointestinal resection on exposure to imatinib. J Clin Oncol 28(9). https://doi.org/10.1200/JCO.2009.26.5785.
Bouchet S, Poulette S, Titier K, Moore N, Lassalle R, Abouelfath A, Molimard M (2016) Relationship between imatinib trough concentration and outcomes in the treatment of advanced gastrointestinal stromal tumors in a real-life setting. Eur J Cancer 57:31–38. https://doi.org/10.1016/j.ejca.2015.12.029
Hølmebakk T, Bjerkehagen B, Boye K, Bruland Ø, Stoldt S, Sundby Hall K (2016) Definition and clinical significance of tumor rupture in gastrointestinal stromal tumors of the small intestine. Br J Surg 103(6):684–691. https://doi.org/10.1002/bjs.10104
Bauer S, Rutkowski P, Hohenberger P, Miceli R, Fumagalli E, Siedlecki JA, Gronchi A (2014) Long-term follow-up of patients with GIST undergoing metastasectomy in the era of imatinib–analysis of prognostic factors (EORTC-STBSG collaborative study). EurJ Surg Oncol 40(4):412–419. https://doi.org/10.1016/j.ejso.2013.12.020
Kurokawa Y, Yang HK, Cho H, Ryu MH, Masuzawa T, Park SR, Kang YK (2017) Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumors of the stomach. Br J Cancer 117(1):25–32. https://doi.org/10.1038/bjc.2017.144
Rutkowski P, Gronchi A, Hohenberger P, Bonvalot S, Schöffski P, Bauer S, Van Coevorden F (2013) Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol 20(9):2937–2943. https://doi.org/10.1245/s10434-013-3013-7
Liu H, Yan Z, Liao G, Yin H (2014) Treatment strategy of rectal gastrointestinal stromal tumor (GIST). J Surg Oncol 109(7):708–713. https://doi.org/10.1002/jso.23562
Huynh TK, Meeus P, Cassier P, Bouché O, Lardière-Deguelte S, Adenis A, Duffaud F (2014) Primary localized rectal/pararectal gastrointestinal stromal tumors: results of surgical and multimodal therapy from the French Sarcoma group. BMC Cancer 14(1):1–9. https://doi.org/10.1186/1471-2407-14-156
Eisenberg BL, Harris J, Blanke CD, Demetri GD, Heinrich MC, Watson JC, Von Mehren M (2009) Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol 99(1):42–47. https://doi.org/10.1002/jso.21160
Le Cesne A, Van Glabbeke M, Verweij J, Casali PG, Findlay M, Reichardt P, Blay JY (2009) Absence of progression as assessed by response evaluation criteria in solid tumors predicts survival in advanced GI stromal tumors treated with imatinib mesylate: the intergroup EORTC-ISG-AGITG phase III trial. J Clin Oncol 27(24):3969. https://doi.org/10.1200/JCO.2008.21.3330
Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Benjamin RS (2007) Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 25(13):1753–1759. https://doi.org/10.1200/JCO.2006.07.3049
Huss S, Pasternack H, Ihle MA, Merkelbach-Bruse S, Heitkötter B, Hartmann W, Wardelmann E (2017) Clinicopathological and molecular features of a large cohort of gastrointestinal stromal tumors (GISTs) and review of the literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rare events. Hum Pathol 62:206–214. https://doi.org/10.1016/j.humpath
Huss S, Elges S, Trautmann M, Sperveslage J, Hartmann W, Wardelmann E (2015) Classification of KIT/PDGFRA wild-type gastrointestinal stromal tumors: implications for therapy. Expert Rev Anticancer Ther 15(6):623–628. https://doi.org/10.1586/14737140.2015.1032941
Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Rutkowski P (2012) Risk of recurrence of gastrointestinal stromal tumor after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 13(3):265–274. https://doi.org/10.1016/S1470-2045(11)70299-6
Acknowledgements
The authors are grateful to their patients and colleagues at the Oncology Center, Mansoura University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Amr Abouzid. The first draft of the manuscript was written by Amr Abouzid and all authors commented on previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Approval was obtained from the Institutional Research Board (IRB) of the Faculty of Medicine, Mansoura University, code R.21.02.1229. Written informed consent was obtained from all the patients included in this study.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abouzid, A., Setit, A., Emarah, Z. et al. Surgical and Oncological Outcomes after Neoadjuvant Therapy for Non-Metastatic Gastric GISTs. Indian J Surg Oncol 14, 21–27 (2023). https://doi.org/10.1007/s13193-022-01611-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-022-01611-w