Abstract
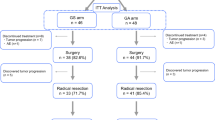
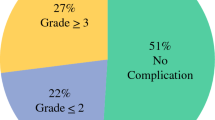
Evaluation of the efficacy of the combination of radical surgery, hyperthermic intraperitoneal chemotherapy (HIPEC), and adjuvant systemic chemotherapy (ACT) in reducing gastric cancer progression in patients with resectable serosa-invasive gastric cancer in a single institution. In 2015–2016, 19 patients with gastric cancer (stage IIB-IIIC) were included in the trial. The trial protocol comprised radical surgery, HIPEC (cisplatin 50 mg/m2 + doxorubicin 50 mg/m2, 42 °C, 1 hour), and 1–8 cycles of ACT (oxaliplatin 100 mg/m2 administered on day 1 of each cycle and oral capecitabine 1000 mg/m2 (or tegafur 10–15 mg/kg) administered twice daily on days 1–14 of each cycle with an interval of 7 days between cycles). Following the ACT treatment, the patients were divided into 2 subgroups—those who underwent up to 6 ACT cycles (1–6 cycles, subgroup ≤ 6–8 patients) and those who underwent 7–8 ACT cycles (subgroup > 6–11 patients). Three-year metastasis-free survival (MFS) for the > 6 subgroup was 91 ± 9%. With a follow-up median of 17 months, 3-year MFS for the ≤ 6 subgroup was not reached − plog-rank = 0.003. The trial showed that in managing advanced gastric cancer patients (pT4a-4bN0-3 M0) by supplementing radical surgery with ACT-enhanced hyperthermic intraperitoneal chemotherapy, ACT proved to be highly effective when administered in its full mode of 7–8 cycles compared with its truncated variant of 1–6 cycles.


Similar content being viewed by others
References
Sugarbaker PH (2006) Adjuvant intraperitoneal chemotherapy for advanced primary gastric cancer. Scand J Surg 95(4):270–273
Coccolini F, Cotte E, Glehen O, Lotti M, Poiasina E, Catena F, Yonemura Y, Ansaloni L (2014) Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol 40:12–26
Xue S-L, Su H-F, Hu X-Q et al (2012) Adjuvant combined systemic chemotherapy and intraperitoneal chemotherapy for locally advanced gastric cancer. Oncol Lett 4:1309–1314
Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA, Gynecologic Oncology Group (2006) Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354:34–43
Reutovich MY, Krasko OV, Sukonko OG (2019) Hyperthermic intraperitoneal chemotherapy in serosa-invasive gastric cancer patients. Eur J Surg Oncol 45(12):2405–2411
Therneau T (2015) _A package for survival analysis in S_. version 2.38. https://CRAN.R-project.org/package=survival. Accessed 12 Dec 2018.
Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, Lee JI, Shin DB, Ji J, Chen JS, Lim Y, Ha S, Bang YJ, CLASSIC trial investigators (2014) Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomized controlled trial. Lancet 15(12):1389–1396
Lee J, Lim DH, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH, Bae JM, Ahn YC, Sohn I, Jung SH, Park CK, Kim KM, Kang WK (2012) Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 30(3):268–273
Kim IH, Park SS, Lee CM, Kim MC, Kwon IK, Min JS, Kim HI, Lee HH, Lee SI, Chae H (2018) Efficacy of adjuvant S-1 versus XELOX chemotherapy for patients with gastric cancer after D2 lymph node dissection: a retrospective, multi-center observational study. Ann Surg Oncol 25(5):1176–1183
Zuo Y, Xu M, Shen D et al (2004) Postoperative intraperitoneal hyperthermic chemoperfusion combined with intravenous chemotherapy for 82 advanced gastric cancer patients. Zhonghua Zhong Liu Za Zhi 26(4):247–249
Shi C, Yang B, Chen Q, Yang J, Fan N (2011) Retrospective analysis of adjuvant intraperitoneal chemotherapy effect prognosis of resectable gastric cancer. Oncology 80(5–6):289–295
Costa WL Jr, Coimbra FJ, Ribeiro HS et al (2012) Safety and preliminary results of perioperative chemotherapy and hyperthermic intraperitoneal chemotherapy (HIPEC) for high-risk gastric cancer patients. World J Surg Oncol 10:195
Glehen O, Passot G, Villeneuve L, Vaudoyer D, Bin-Dorel S, Boschetti G, Piaton E, Garofalo A (2014) GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC Cancer 14:183
Porschen RJ, Arkenau HT, Kubicka S, Greil R, Seufferlein T, Freier W, Kretzschmar A, Graeven U, Grothey A, Hinke A, Schmiegel W, Schmoll HJ, AIO Colorectal Study Group (2007) Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO colorectal study group. J Clin Oncol 25(27):4217–4223
Cho EK, Lee WK, Im SA, Lee SN, Park SH, Bang SM, Park DK, Park YH, Shin DB, Lee JH (2005) A phase II study of epirubicin, cisplatin and capecitabine combination chemotherapy in patients with metastatic or advanced gastric cancer. Oncology 68(4–6):333–340
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR, Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom (2008) Capecitabine and Oxaliplatin for advanced Esophagogastric Cancer. N Engl J Med 358:36–46
Kim GM, Jeung HC, Rha SY, Kim HS, Jung I, Nam BH, Lee KH, Chung HC (2012) A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur J Cancer 48(4):518–526
Fuse N, Bando H, Chin K, Ito S, Yoshikawa T, Tsuburaya A, Terashima M, Kawashima Y, Fukunaga T, Gotoh M, Emi Y, Yoshida K, Oki E, Takahashi S, Kuriki H, Sato K, Sasako M (2017) Adjuvant capecitabine plus oxaliplatin after D2 gastrectomy in Japanese patients with gastric cancer: a phase II study. Gastric Cancer 20(2):332–340
Sehnake KJ, Sugarbaker PH, Yoo D (1999) Neutropenia following perioperative intraperitoneal chemotherapy. Tumori 85(1):41–46
Borner MM, Schoffski P, de Wit R et al (2002) Patient preference and pharmacokinetics of oral modulated UFT vs intravenous fluorouracil and leucovorin: a randomized crossover trial in advanced colorectal cancer. Eur J Cancer 38(3):349–358
Acknowledgments
The authors thank all patients, coordinators, and investigators who participated in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
“All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (include name of committee + reference number) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reutovich, M.Y., Krasko, O.V. & Sukonko, O.G. Efficacy of Adjuvant Systemic Chemotherapy Combined with Radical Surgery and Hyperthermic Intraperitoneal Chemotherapy in Gastric Cancer Treatment. Indian J Surg Oncol 11, 337–343 (2020). https://doi.org/10.1007/s13193-020-01102-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-020-01102-w