Abstract
Introduction
Carbon monoxide (CO) is a colorless and odorless gas that is a leading cause of environmental poisoning in the USA with substantial mortality and morbidity. The mechanism of CO poisoning is complex and includes hypoxia, inflammation, and leukocyte sequestration in brain microvessel segments leading to increased reactive oxygen species. Another important pathway is the effects of CO on the mitochondria, specifically at cytochrome c oxidase, also known as Complex IV (CIV). One of the glaring gaps is the lack of rigorous experimental models that may recapitulate survivors of acute CO poisoning in the early phase. The primary objective of this preliminary study is to use our advanced swine platform of acute CO poisoning to develop a clinically relevant survivor model to perform behavioral assessment and MRI imaging that will allow future development of biomarkers and therapeutics.
Methods
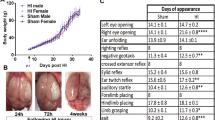
Four swine (10 kg) were divided into two groups: control (n = 2) and CO (n = 2). The CO group received CO at 2000 ppm for over 120 min followed by 30 min of re-oxygenation at room air for one swine and 150 min followed by 30 min of re-oxygenation for another swine. The two swine in the sham group received room air for 150 min. Cerebral microdialysis was performed to obtain semi real-time measurements of cerebral metabolic status. Following exposures, all surviving animals were observed for a 24-h period with neurobehavioral assessment and imaging. At the end of the 24-h period, fresh brain tissue (cortical and hippocampal) was immediately harvested to measure mitochondrial respiration.
Results
While a preliminary ongoing study, animals in the CO group showed alterations in cerebral metabolism and cellular function in the acute exposure phase with possible sustained mitochondrial changes 24 h after the CO exposure ended.
Conclusions
This preliminary research further establishes a large animal swine model investigating survivors of CO poisoning to measure translational metrics relevant to clinical medicine that includes a basic neurobehavioral assessment and post exposure cellular measures.





Similar content being viewed by others
References
Ran T, Nurmagambetov T, Sircar K. Economic implications of unintentional carbon monoxide poisoning in the United States and the cost and benefit of CO detectors. Am J Emerg Med. 2018;36(3):414–9. https://doi.org/10.1016/j.ajem.2017.08.048.
Weaver LK, Hopkins RO, Larson-Lohr V. Neuropsychologic and functional recovery from severe carbon monoxide poisoning without hyperbaric oxygen therapy. Ann Emerg Med. 1996;27(6):736–40. https://doi.org/10.1016/s0196-0644(96)70192-0.
Pepe G, Castelli M, Nazerian P, Vanni S, Del Panta M, Gambassi F, et al. Delayed neuropsychological sequelae after carbon monoxide poisoning: predictive risk factors in the Emergency Department A retrospective study. Scand J Trauma, Resusc Emerg Med. 2011;19:16.
Penney DG. Acute carbon monoxide poisoning: animal models: a review. Toxicology. 1990;62(2):123–60. https://doi.org/10.1016/0300-483x(90)90106-q.
Gandini C, Castoldi AF, Candura SM, Locatelli C, Butera R, Priori S, et al. Carbon monoxide cardiotoxicity. J Toxicol Clin Toxicol. 2001;39(1):35–44. https://doi.org/10.1081/clt-100102878.
Rose JJ, Wang L, Xu Q, McTiernan CF, Shiva S, Tejero J, et al. Carbon Monoxide Poisoning: Pathogenesis, Management, and Future Directions of Therapy. Am J Respir Crit Care Med. 2017;195(5):596–606. https://doi.org/10.1164/rccm.201606-1275CI.
Akyol S, Erdogan S, Idiz N, Celik S, Kaya M, Ucar F, et al. The role of reactive oxygen species and oxidative stress in carbon monoxide toxicity: an in-depth analysis. Redox Rep. 2014;19(5):180–9. https://doi.org/10.1179/1351000214Y.0000000094.
Jang DH, Kelly M, Hardy K, Lambert DS, Shofer FS, Eckmann DM. A preliminary study in the alterations of mitochondrial respiration in patients with carbon monoxide poisoning measured in blood cells. Clin Toxicol (Phila). 2017;55(6):579–84. https://doi.org/10.1080/15563650.2017.1288912.
Li CX, Patel S, Wang DJ, Zhang X. Effect of high dose isoflurane on cerebral blood flow in macaque monkeys. Magn Reson Imaging. 2014;32(7):956–60. https://doi.org/10.1016/j.mri.2014.04.019.
Jang DH, Piel S, Greenwood JC, Kelly M, Mazandi VM, Ranganathan A, et al. Alterations in cerebral and cardiac mitochondrial function in a porcine model of acute carbon monoxide poisoning. Clin Toxicol (Phila). 2021;59(9):801–9. https://doi.org/10.1080/15563650.2020.1870691.
Lewis AT, Forti RM, Alomaja O, Mesaros C, Piel S, Greenwood JC, et al. Preliminary Research: Application of Non-Invasive Measure of Cytochrome c Oxidase Redox States and Mitochondrial Function in a Porcine Model of Carbon Monoxide Poisoning. J Med Toxicol. 2022;18(3):214–22. https://doi.org/10.1007/s13181-022-00892-5.
Zhang Y, Xie Z. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol. 2012;72(4):630. https://doi.org/10.1002/ana.23683.
Greenwood JC, Talebi FM, Jang DH, Spelde AE, Tonna JE, Gutsche JT, et al. Topical nitroglycerin to detect reversible microcirculatory dysfunction in patients with circulatory shock after cardiovascular surgery: an observational study. Sci Rep. 2022;12(1):15257. https://doi.org/10.1038/s41598-022-19741-0.
Greenwood JC, Talebi FM, Jang DH, Spelde AE, Kilbaugh TJ, Shofer FS, et al. Protocol for the MicroRESUS study: The impact of circulatory shock and resuscitation on microcirculatory function and mitochondrial respiration after cardiovascular surgery. PLoS ONE. 2022;17(8):e0273349. https://doi.org/10.1371/journal.pone.0273349.
Jang DH, Greenwood JC, Owiredu S, Ranganathan A, Eckmann DM. Mitochondrial networking in human blood cells with application in acute care illnesses. Mitochondrion. 2017. https://doi.org/10.1016/j.mito.2017.12.009.
Jang DH, Greenwood JC, Spyres MB, Eckmann DM. Measurement of Mitochondrial Respiration and Motility in Acute Care: Sepsis, Trauma, and Poisoning. J Intensive Care Med. 2017;32(1):86–94. https://doi.org/10.1177/0885066616658449.
Mavroudis CD, Mensah-Brown KG, Ko TS, Boorady TW, Massey SL, Abend NS, et al. Electroencephalographic Response to Deep Hypothermic Circulatory Arrest in Neonatal Swine and Humans. Ann Thorac Surg. 2018;106(6):1841–6. https://doi.org/10.1016/j.athoracsur.2018.06.036.
Helke KL, Swindle MM. Animal models of toxicology testing: the role of pigs. Expert Opin Drug Metab Toxicol. 2013;9(2):127–39. https://doi.org/10.1517/17425255.2013.739607.
Sullivan S, Friess SH, Ralston J, Smith C, Propert KJ, Rapp PE, et al. Improved behavior, motor, and cognition assessments in neonatal piglets. J Neurotrauma. 2013;30(20):1770–9. https://doi.org/10.1089/neu.2013.2913.
Rath E, Haller D. Mitochondria at the interface between danger signaling and metabolism: role of unfolded protein responses in chronic inflammation. Inflamm Bowel Dis. 2012;18(7):1364–77. https://doi.org/10.1002/ibd.21944.
Yamada T, Hisanaga M, Nakajima Y, Kanehiro H, Aomatsu Y, Ko S, et al. The serum interleukin 8 level reflects hepatic mitochondrial redox state in hyperthermochemohypoxic isolated liver perfusion with use of a venovenous bypass. Surgery. 1999;125(3):304–14.
Hoffmann RF, Jonker MR, Brandenburg SM, de Bruin HG, Ten Hacken NHT, van Oosterhout AJM, et al. Mitochondrial dysfunction increases pro-inflammatory cytokine production and impairs repair and corticosteroid responsiveness in lung epithelium. Sci Rep. 2019;9(1):15047. https://doi.org/10.1038/s41598-019-51517-x.
Funding
This publication was made possible by P30 ES013508 from the National Institute of Environmental Health Sciences, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIEHS, NIH.
1. R21ES031243 (Jang).
2. R03HL154232 (Jang).
3. R56HL158696 (Jang).
4. R01HL166592 (Jang).
5. R01HL141386 (Kilbaugh).
6. R01NS113945 (Baker).
7. Thomas B. McCabe and Jeannette E. Laws McCabe Fund Pilot Award (Mavroudis).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
None.
Additional information
Supervising Editor: Mark B. Mycyk, MD.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mavroudis, C.D., Lewis, A., Greenwood, J.C. et al. Investigation of Cerebral Mitochondrial Injury in a Porcine Survivor Model of Carbon Monoxide Poisoning. J. Med. Toxicol. 20, 39–48 (2024). https://doi.org/10.1007/s13181-023-00971-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13181-023-00971-1