Abstract
Cardiac diseases are the leading cause of death and reach epidemic proportions with aging. Advanced heart disease results from an abrupt or progressive loss of contractile cardiomyocytes. Following percutaneous coronary intervention and revascularization regenerative medicine aims at effectively repair damaged tissue and replacement of lost cardiomyocytes. However, mixed results were obtained from trials using bone marrow-derived stem cells. Benefits were rather attributed to paracrine effects leading to inhibition or reverse of negative remodeling processes than to regeneration of viable cardiomyocytes. Thus the aim of regenerative medicine, in particular stem cell research, to generate viable cardiac muscle has so far not been achieved in humans, reflecting our incomplete understanding of underlying biological mechanisms. Moreover, there is growing evidence that substantial person-to-person differences in the outcome of stem cell therapy exists. We here review our present knowledge in evolving stem cell based cardiovascular medicine and highlight personalized aspects of stem cell interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of human stem cells is becoming pivotal for the development of new therapeutic strategies for many organ-specific diseases, which are characterized by abrupt or progressive loss of function and for which existing therapies are not satisfactory. Although state-of-the-art interventional and medical therapy for myocardial infarction (MI) and heart failure where implemented, clinical outcome remains poor in post-MI patients with reduced left ventricular (LV) function. The 1-year mortality rate is 13%, and the incidence of the combined endpoint of death, recurrent infarction, or hospitalization for heart failure is 26% [1]. The majority of treatments currently in use are straightened towards the general population. In contrast personalized medicine is based on targeted therapeutic approaches that allow patient specific care [2] in order to maximize the therapeutic potential while minimizing the risk of adverse effects. Regenerative medicine, cellular therapy in particular, warrants a personalized approach because of the multitude of interactions between donor and host that can decisively influence treatment outcomes [3]. Therefore, the precise planning of stem cell therapy must be coordinated ideally with the characteristics of individual patient profiles to fully harness its therapeutic potential [4].
Given the aging of the western world population and the increase in the prevalence of cardiovascular risk factors such as obesity and type 2 diabetes, cardiovascular diseases will continue to be a significant health concern in the ongoing century [5, 6]. These trends suggest an unmet need for therapies to regenerate or repair damaged cardiac tissue. After first promising results from stem and progenitor cell transplantation in small animal models of cardiac ischemic injury clinical researchers launched the exploration of cell transfer strategies in acute and chronic ischemic human heart disease (Fig. 1). In the emerging field of stem cell research and therapy heterogeneous populations of stem and progenitor cells residing in bone marrow (BM), adipose tissue, and skeletal muscle or circulating in the blood showed potential to be capable of improving myocardial function (Fig. 1). Based on experimental data demonstrating that infusion or injection of stem/progenitor cells derived from various sources enhance blood flow, neovascularization and improve heart function after myocardial infarction [7], clinical trials were started about a decade ago to treat patients with cardiac ischemia [8, 9]. The primary goal of all studies using stem and progenitor cells was to improve myocardial function and reduce remodeling by replacement of the fibrotic scar tissue with viable cardiac myocytes (Fig. 2). Initial trials of autologous (BM)-derived stem cells (BMC) documented safety and feasibility both in patients with acute myocardial infarction and in those with chronic ischemia and reported a modest, beneficial effect on LV function [10–13]. The relatively small number of patients, the absence of a control group undergoing repeat coronary infusions, and the relatively mild improvements in LV function after the index infarction challenged distinct interpretation of these results concerning identification of patient populations with particular benefit of stem cell interventions.
Human stem cells and stem cell sources for cell-based functional repair after myocardial ischemia. Patient-specific autologous approach by reprogrammed induced pluripotent stem cells or multi- or monopotent adult stem cells compared to an allogenic approach using pluripotent embryonic stem cells is depicted. Stem cells from different sources can be expanded in vitro and differentiated into cardiovascular progenitor cells and mature cardiovascular cells (e.g. cardiomyocytes, red: α-actinin; endothelial cells, yellow: flt-1; smooth muscle cells, green: α-smooth muscle actin). Monopotent skeletal myoblasts proliferate and form multinucleated myotubes (myotube, green: titin). Following PCI for revascularization cells will be applied to the side of injury. Protocols in active randomized clinical trials are ongoing to address issues of optimal timing, dose and route of cell delivery. Abbreviations: PCI percutaneous coronary intervention, CSC cardiac stem cells; BMC bone marrow cell, EPC endothelial progenitor cell, MSC mesenchymal stem cell, SkM skeletal myoblast
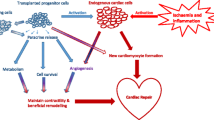
Stem cell triggered myocardial repair via cardioprotection and cardiomyocytes regeneration. Functional benefits obtained from trials using bone marrow-derived stem cells were rather attributed to trophic-paracrine effects (cardiprotection) leading to inhibition or reverse of negative remodeling processes than to regeneration of viable cardiomyocytes (cardiac regeneration)
Clinical experience of double-blind randomized placebo-controlled studies
Two subsequent double-blind randomized placebo-controlled studies, Leuven-AMI and REPAIR-AMI (Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction) investigated a similar AMI population using comparable BMC isolation, preparation, and characterization protocols [14, 15]. Thereby addressing some of these confounding variables associated with bone marrow aspiration and a second catheterization [16, 17]. Within clinical trials, the largest randomized, controlled clinical study of BMC therapy, the REPAIR-AMI trial, observed improvement in left ventricular ejection fraction (LVEF) of 2.5% in patients after MI, while the Leuven-AMI trial found an improved LVEF of 1.2%. In addition, patients receiving cell transfer in the Leuven-AMI trial had a significantly greater reduction in infarct size for a similar area at risk, as assessed using repeated MRI, and a greater recovery of regional systolic function. Importantly, these beneficial effects were sustained at 1-year follow-up [18]. Although these potential effects of BMC therapy on LV function are less than many investigators were hoping for, it should be noted that several of our established clinical therapies with an impact on prognosis in patients with MI and a reduced LV function, such as angiotesinconverting enzyme (ACE) inhibitor or ß-blocker therapy, are associated with similar improvements in LVEF and have been observed in patients after MI with a less optimal background therapy as compared with present studies [19].
Population with most benefit of autologous BM-derived stem cell (BMC) therapy
Within the REPAIR-AMI trial cell transfer was not always performed on the same day after the index percutaneous coronary intervention but ranged from 3 to 7 days, whereas patients in the Leuven-AMI trial received placebo or BMC infusion at 24 h after the first intervention. Interestingly, when data were stratified by time of cell transfer and by severity of LV dysfunction at baseline, the benefit was predominantly observed in patients receiving delayed cell transfer enabling ex vivo expansion of cells and autologous transplantation. Moreover, patients with a baseline LVEF below the median value of 49% showed significantly enhanced metabolic recovery receiving cell transfer. Although the studies were not powered to primarily evaluate the effects of MI size, there was a significant interaction between infarct severity and subsequent benefit from cell transfer [20–22]. The best results were observed in patients suffering from large infarctions (anterior infarction and significant LV dysfunction) with more depressed global ejection fractions. Concerning the timing of cell transplantation after MI it has been speculated that by giving the cells sooner after the index infarction the high incidence of microvascular obstruction that is observed in the early phase of reperfusion may limit homing, engraftment, and survival of infused cells.
The large number of patients enrolled in the REPAIR-AMI trial allowed to test several predefined secondary endpoints in order to generate hypotheses for the next generation of clinical trials. Indeed, the beneficial effect on clinical endpoints was also preferentially observed in those patients with a lower baseline ejection fraction. Therefore, these analyses are generating the hypothesis that selecting patients with severe impairment of LVEF after MI maybe one way to increase the benefit of this therapy. This focus is consistent with the unmet clinical need in this expanding population of ischemic cardiomyopathy and represents the prime target in next-generation clinical trials.
The degree of improvement of myocardial contractility and perfusion observed in these trials depend substantially on the functional quality and number of progenitor and stem cells that can be harvested from the individual patient [23–26]. This in turn is dependent on age, comorbidities, medications and other factors. Numerous experiments have shown that in patients with diabetes there is a functional impairment of the BM-derived endothelial progenitor cells (EPC) and a lower number of circulating pluripotent cells in comparison to patients without diabetes. In addition, patients with ischemic cardiomyopathy have fewer EPC in the BM, while the migratory capacity of these cells is significantly impaired [27]. Other studies have demonstrated shortening of the telomeres in progenitor cells isolated from the BM and peripheral blood in patients with coronary heart disease [28, 29]. Therefore, if autologous cells are used for myocardial repair their functional status, which is dependent on the individual risk profile of the patients, might profoundly affect the outcome. Because the functional impairment of BM-derived cells harvested from patients with chronic heart failure may limit the efficiency of such treatment, and since genetic engineering is not always possible, another way to overcome the obstacles would be to use allogeneic BM-cells isolated from healthy subjects. This requires isolation and expansion of immunologically compatible cells suitable for replacement therapies in senescent adults.
Transplantation of allogenic derived stem cells
Comparisons between allogenic and autologous transplant therapies have demonstrated the critical role of the immune system in determining clinical outcomes. Autologous transplantations employ a patients own tissue and thus present no tissue incompatibility between the donor and the host due to a lack of stimulation of the immune response. However, in cases where there is a need for immediate treatment with stem cells, it will not be practical to wait for ex vivo expansion and re-transplantation of autologous stem cells [30]. Moreover, because the functional impairment of stem and progenitor-derived cells harvested from patients with chronic heart failure may limit the efficiency of autologous stem cell treatment. The use of allogeneic stem cells isolated from healthy subjects might offer a promising alternative approach.
Mesenchymal (bone marrow stromal) cells (MSC)
MSC are so far the best candidate for this approach, because they are immunoprivileged and escape rejection by the release of immunomodulatory factors and inhibition of T-cell proliferation. MSC are precursors of non-hematopoietic tissues (e.g., muscle, bone, tendons, ligaments, adipose tissue, and fibroblasts) that are obtained relatively easily from autologous BM. MSC make up a small proportion of BM cells (0.001–0.01% of nucleated cells in the bone marrow), but can be harvested from the BM and even more efficiently from the adipose tissue (ca. 1 million MSC per 100 cc of fat tissue). Importantly, the cells can be expanded in vitro and stored [31, 32]. This would allow an available treatment of patients with acute MI and severe left ventricular dysfunction, without the need to wait for the cell processing and expansion. The potential of MSC to undergo cardiomyocyte differentiation is still under dispute, but several experimental and clinical trials have shown their potential to improve LV function in ischemic cardiomyopathy. Because of their ability to undergo expansion, MSC can also be bioengineered to overexpress factors that increase their engraftment and differentiation [33]. Several animal model studies have shown that treatment with MSC significantly increases myocardial function and capillary formation [34]. A randomized clinical trial implanting MSC after MI has demonstrated significant improvement in global and regional LV function, and clinical trials are currently underway to investigate the application of allogeneic and autologous MSC for acute MI and myocardial ischemia, respectively [13].
Heterogeneity among BMC transplanted stem cells
Initial trials of autologous BMC transfer have either used unfractionated or mononuclear cell fractions in patients after MI. BMC clearly represent a heterogeneous population of cells, so that it remains unclear which of the cells is particularly important for the potential effects on cardiac repair. The pool of mononuclear cells isolated from the BM by gradient centrifugation contains mostly committed cells, a small number of monopotent progenitor cells, and even fewer bona fide stem cells. Stem cells are defined as cells that have the capacity to self renew, are multipotent/pluriopotent, can be clonally expanded, and are divided into embryonic stem cells and adult stem cells. In addition, progenitor cells comprise cells which are already more committed. Traditional views in stem cell biology held that one particular tissue type arises from one particular stem cell. As evidence of stem cell plasticity amounts, the traditional views are fading away in favor of more complex themes involving cross functions of stem cells. The effects of environmental factors on stem and progenitor cell behaviour are diverse and can include alteration of gene expression within distinct populations. A single standard method of culturing and expansion stem and progenitor cells has not been established, and therefore comparison of results of stem cell therapies may be difficult to interpret based on different culture conditions. Furthermore the BM microenvironment varies from person to person due to multiple factors, including genomic differences and overall health. Despite the influence of culture conditions, stem cells show donor differences with regard to the gene expression, although similar isolation protocols and variations can emerge when cultures become confluent or are expanded by serial passage while they approach senescence [35]. Therefore, generalizations about the effect of stem cells are difficult until variations among cell types, culture conditions, donors and hosts are elucidated.
Mechanism of action
Despite rejection reactions of the immune system there are several other reasons to suggest that the implementation of personalized medicine with stem cells is required. Stem and progenitor cells have particular niches in which they stay until mobilization and to which they home in case of injury. Several humoral factors govern and control these processes [36]. Thus variations among microenvironments which could be influenced by the patient’s background as well as other underlying clinical disorders might be relevant for the functions of stem cells in each different host [30]. The composition of the microenvironment and the presence of certain soluble factors are major determinants for proliferation, differentiation and homing of applied stem or progenitor cells. Knowledge on cytokines and growth factors present in injured myocardium is limited. However, functions of transplanted stem cell will depend on the discretion of paracrine factors which may result in beneficial or undesirable outcomes. Therefore identification of these factors, by secretome analyses and bioinformatic approaches, will advance cell- and protein-based therapies to promote healing and inhibit pathological remodeling of the heart after ischemia. So far, the results obtained in vitro do not ensure that applied stem cells will provide the desired therapeutic result in vivo. In vitro findings must be confirmed and verified in vivo before translation to patients. It is critical to conduct in vitro stem cell research with caution because challenges can frequently re-appear when transplantation is conducted in humans. Understanding of the microenvironment factors of injured myocardium at the relevant time must be ascertained in-depth in order to optimize homing and differentiation processes. Moreover, timeline, dose and route of application are critical for stem cells to exert maximal beneficial effects, but current evidence is not well supported regarding when particular signals are present in injured tissue in order to have optimal clinical benefits.
As basic science findings and knowledge on stem cell biology continue to accumulate, questions are now emerging on how to translate the existing stem cell research data towards therapeutic applications that have predictable and adequate clinical effectiveness. The entire process, from patient profiling to delivery and maintenance of successful stem cell therapy, will therefore require the understanding of numerous factors that impact research and treatment outcomes. The abundance of biological properties inherent to stem and progenitor cells requires consideration of all possible interactions they can have with the host.
Given the above argument, it appears that personalized treatment regimens would require an understanding of the molecular and cellular interactions with the prospective microenvironment. Through this information, therapeutic outcome of stem cell therapies would be better predicted. Patient specific genomic profiling and molecular understanding of stem cell biology are therefore the major tasks for clinicians and scientists in order to reach desirable results in future approaches, reminding us that a complete understanding of basic science is fundamental prior to and during implementation of these cell-based interventions.
Nonetheless, the field is still evolving, and single clinical failures should not interfere with the overarching favorable evidence that has accumulated thus far.
Other stem cells regenerating damaged myocardium and personalized treatments
The source of stem cells must be carefully chosen on functional and physical criteria that lead to optimal outcomes. The use of tissue-specific stem cells for repair has been traditionally attempted. Despite unipolar progenitor cells multipotent stem cells have been shown to facilitate the recovery of different tissue types. As mentioned above, many types of stem cells have been applied to regenerate damaged myocardium and demonstrated sufficient promise to further explorate them in large-scale, controlled clinical trials (Table 1). However, the lack of transdifferentiation of BMC into cardiomyocytes and the absence of standardized protocols has made it difficult to compare and contextualize the results generated by the various trials. Most studies published to date have enrolled fewer than 25 patients, and the studies vary in terms of cell types and preparations used, methods of delivery, patient populations, and trial outcomes. However, the mixed results that have been observed in these studies do not necessarily argue against using stem cells for cardiac repair. Rather, preliminary results illuminate the many gaps in understanding of the mechanisms by which these cells regenerate myocardial tissue and argue for improved characterization of cell preparations and delivery methods to support clinical applications.
Mesenchymal (bone marrow stromal) cells (MSC)
MSC are so far the best candidate for this approach, because they are immunoprivileged and escape rejection by the release of immunomodulatory factors and inhibition of T-cell proliferation. MSC are precursors of non-hematopoietic tissues (e.g., muscle, bone, tendons, ligaments, adipose tissue, and fibroblasts) that are obtained relatively easily from autologous BM. MSC make up a small proportion of BM cells (0.001–0.01% of nucleated cells in the bone marrow), but can be harvested from the BM and even more efficiently from the adipose tissue (ca. 1 million MSC per 100 cc of fat tissue). Importantly, the cells can be expanded in vitro and stored [31, 32]. This would allow an available treatment of patients with acute MI and severe left ventricular dysfunction, without the need to wait for the cell processing and expansion. The potential of MSC to undergo cardiomyocyte differentiation is still under dispute, but several experimental and clinical trials have shown their potential to improve LV function in ischemic cardiomyopathy. Because of their ability to undergo expansion, MSC can also be bioengineered to overexpress factors that increase their engraftment and differentiation [33]. Several animal model studies have shown that treatment with MSC significantly increases myocardial function and capillary formation [34]. A randomized clinical trial implanting MSC after MI has demonstrated significant improvement in global and regional LV function, and clinical trials are currently underway to investigate the application of allogeneic and autologous MSC for acute MI and myocardial ischemia, respectively [13].
Embryonic stem cells (ESC)
Because ESC cells are pluripotent, they can potentially give rise to a variety of cell types that are instrumental in regenerating damaged myocardium, including cardiomyocytes, endothelial cells, and smooth muscle cells (Fig. 1). To this end, mouse and human ESC have been shown to differentiate spontaneously to form endothelial and smooth muscle cells in vitro and in vivo, and human ESC differentiate into myocytes with the structural and functional properties of cardiomyocytes [37, 38]. Moreover, ESC that were transplanted into ischemically-injured myocardium in rats differentiated into normal myocardial cells that remained viable for up to 4 months, suggesting that these cells may be candidates for regenerative therapy in humans [39].
Although the potential benefits of personalized stem cell therapy are vast, as discussed above, implementation of ESC in personalized therapy must first overcome other significant obstacles. First, perhaps the most obvious challenges to personalized therapy for ESC revolve around immunogenicity, which can lead to rejection of implanted cells. ESC are immunogenic as evident by MHC expression, and MHC expression is increased when ESC lose their pluripotency [40]. Hence, MHC antigens must be matched as closely as possible to minimize rejection [40]. In order to serve as stem cell therapy autologous approaches with individual ESC would have been to be generated for diverse patient populations [41]. The generation of such a large number of ESC lines would be impractical considering the ethical and scientific limitations of harvesting these cells. Creation of a reliable source of stem cells may be more difficult than previously thought [42].
Second, to ensure delivery of healthy stem cells, the cell population must be purified prior to delivery into the host because mutations in the genome or undifferentiated ESC can lead to development of tumors, such as teratomas [40]. However, the purification process may induce unforeseen genetic damage, and quality control must be performed before delivery to the host. Transmission of infection by ESC may also limit their use [41].
Aside from the biological challenges to the employment of ESC in personalized medicine, numerous ethical issues emerge. The source of ESC is the inner cell mass, which is located in the blastocyst of a zygote (Fig. 1). Adult stem cells, on the other hand, bypass these problems because they can be obtained readily from the BM. Thus, therapeutic avenues that arise from adult stem cells may address some of the attendant socio-ethical concerns more effectively compared to ESC investigations.
Skeletal myoblasts (SM)
While SM are committed progenitors of skeletal muscle cells, their autologous origin, high proliferative potential, commitment to a myogenic lineage, and resistance to ischemia promoted their use as the first stem cell type to be explored extensively for cardiac application. Studies in rats and humans have demonstrated that these cells can repopulate scar tissue and improve left ventricular function following transplantation [43]. However, SM-derived myocytes do not function in complete concert with native myocardium. The expression of two key proteins involved in electromechanical cell integration, N-cadherin and connexin 43, are downregulated in differentiated myotubes, and the engrafted cells develop a contractile activity phenotype that appears to be unaffected by neighboring cardiomyocytes [44, 45]. Although stem cells appear to be relatively safe in the majority of recipients to date, an increased frequency of non-sustained ventricular tachycardia, an arrhythmia, has been reported in conjunction with the use of skeletal myoblasts, possibly resulting from the lack of electrical coupling between SM-derived myocytes and native tissue [46, 47]. While this proarrhythmic effect occurs relatively early after cell delivery and does not appear to be permanent, its presence highlights the need for careful safety monitoring when these cells are used. Additionally, animal models have demonstrated that stem cells rapidly diffuse from the heart to other organs (e.g., lungs, kidneys, liver, spleen) within a few hours of transplantation, an effect observed regardless of whether the cells are injected locally into the myocardium [48]. This migration may or may not cause side-effects in patients; however, it remains a concern related to the delivery of all stem cells in humans.
Endothelial progenitor cells (EPC)
The endothelium is a layer of specialized cells that lines the interior surface of all blood vessels including the heart. This layer provides an interface between circulating blood and the vessel wall. EPC are BM-derived stem cells that are recruited into the peripheral blood in response to tissue ischemia [49]. EPC are precursor cells that express some cell-surface markers characteristic of mature endothelium and some of hematopoietic cells [50]. EPC home in on ischemic areas, where they differentiate into new blood vessels. The new vascularization induced by these cells prevents cardiomyocyte apoptosis and LV remodeling, thereby preserving ventricular function [51]. However, no change has been observed in non-infarcted regions upon EPC administration. The majority of clinical trials of human cell therapy for various forms of ischemic vascular disease have relied on autologous bone marrow-derived, CD34+/KDR+ and CD133+ cells that have displayed modest regenerative and reparative roles [52]. The level of circulating CD34 + KDR + EPCs predicted the occurrence of cardiovascular events and death from cardiovascular causes in the analyzed study population [52]. Compared to preclinical studies performed in rodent models clinical trials have not been as efficacious as predicted. Conducted studies have often lacked detailed characterization of cellular function and lineage of origin resulting in the term EPC encompassing different cell populations, including cells of myeloid, lymphoid and endothelial origin. Not surprisingly these putative EPC populations have demonstrated a mixed ability to contribute to the regeneration or formation of blood vessels [53]. Clinical trials are currently underway to assess EPC therapy for growing new blood vessels and regenerating myocardium.
Induced pluripotent stem cells (iPSC)
Recent progress with pluripotent stem cells and particularly the use of iPS cells may enabeling researchers and clinicians to develop patient specific therapies [54]. The major therapeutic advantage of iPS cells over ES cells is related to its source of origin. iPS cells can be derived from almost any somatic tissue providing immunologic compatibility and the lack of ethical controversies in human stem cell research. These cell lines may therefore prove invaluable for therapeutic interventions and treatment of cardiac and vascular diseases either through cell replacement or through secretion of critical factors. These cells are genetically identical to the donor cells [55]. One particularly appealing aspect of iPSCs is that, in theory, they can be directed to differentiate into a specified lineage that will support treatment or tissue regeneration. Jet while iPSCs have great potential as sources of adult mature cells, much remains to be learned about the processes by which these cells differentiate. For example, iPSCs created from human and murine fibroblasts can give rise to functional cardiomyocytes that display hallmark cardiac action potentials [56–59]. The proof-of-concept studies showed that iPS can be efficiently differentiated into cardiac lineage cells that demonstrate the expression of early cardiac markers, followed by cardiac structural proteins, eventually leading to the formation of spontaneously contracting cardiomyocytes coupled by gap junctions. The pattern of the transmembrane calcium currents and action potential characteristics is similar to that of cardiomyocytes derived from embryonic stem cells. However, the maturation process and the electrophysiological properties of the iPS-derived cardiomyocytes were impaired compared to that seen with cardiomyocytes derived from ESC or fetal tissue [57, 60]. Furthermore, variation exists in the expression of genetic markers in the iPSC-derived cardiac cells as compared to that seen in ESC-derived cardiomyocytes. Therefore, iPSC-derived cardiomyocytes demonstrate normal commitment but impaired maturation, and it is unclear whether observed defects are due to technical or biological barriers. Thus, before these cells can be used for therapy, it will be critical to distinguish between iPSC-specific and disease-specific phenotypes.
Unlike adult stem cells, iPS does show a potential to generate tumors, probably due to the insertional mutagenesis, so their use in humans cannot be envisioned in the near future. A possible way to overcome the risk is to use viral-free vectors and the proteins of reprogramming factors instead of DNA encoding [61]. For putative regenerative medicine applications, patient safety is the foremost consideration. Standardized methods must be developed to characterize iPSCs and their derivatives. Furthermore, reprogramming has demonstrated a proof-of-principle, jet the process is currently too inefficient for routine clinical application. Thus, unraveling the molecular mechanisms that govern reprogramming is a critical first step toward standardizing protocols and personalized usage. Based on the exciting developments in this area to date, induced pluripotent stem cells will likely support future therapeutic interventions, either directly or as research tools to establish novel models for degenerative disease, drug screening or cell based therapies.
Despite replacement therapies designed to improve cardiac and vascular function in compromised individual’s iPS technology offers the unprecedented possibility of using human cells to study human diseases for which there are no animal models, such as Brugada syndrome and hypertrophic cardiomyopathy [62]. iPS cells, when produced directly from patients with cardiovascular diseases of unknown etiologies, will lead to the development of new model systems that can be employed to investigate cardiac and vascular developmental defects. This is an important benefit given that many genetic diseases are of a sporadic nature with no family history. More than ten human disease-specific iPS cell lines have been established, ranging from simple single gene deficiencies to complex multifactorial diseases of unknown genetic origin [62]. While much remains to be learned in the field of iPSC research, the development of reprogramming techniques represents a breakthrough that will ultimately open many new avenues of research and therapy.
Resident cardiac stem cells (CSC)
Most of the cell transplantation-based therapeutic approaches used so far are predicated on the concept that the adult human myocardium does not have intrinsic regenerative capacity because the working cardiomyocytes are terminally differentiated cells with no regenerative capacity. However, recent evidence suggests that the adult myocardium contains a small population of endogenous stem cells that most likely facilitate minor repair and turnover-mediated cell replacement [63]. Several groups have identified cardiac stem cells possessing growth factor-receptor systems and reported different membrane markers or transport proteins. These endogenous CSC are able to regenerate the contractile myocytes and endothelial and smooth muscle cells of the microvasculature, but their numbers vary substantially between species, and it is unclear whether they constitute phenotypic variations of a unique cell type [64, 65].
The cells can be harvested in limited quantity from human endomyocardial biopsy specimens and can be injected into the site of infarction to promote cardiomyocyte formation and improvements in systolic function [66, 67]. The problem is that these reservoirs of cells are usually overridden in patients with AMI, advanced coronary artery disease, and chronic heart failure. Despite this limited capacity for regeneration of myocardium, the existence of these repair mechanisms suggests that cardiac repair may be achieved therapeutically in these clinical settings, given the appropriate stimulation (in situ activation, multiplication and differentiation of the eCSC) and/or adoptive transfer of (stem) cells involved in these processes [68, 69]. Cardiac repair via endogenous CSC represents a major target for translational research in the years to come.
Non-biological challenges of stem cell therapies
Non-biological concerns include socio-political factors that may hinder the implementation of personalized medicine in cellular replacement therapies. The premium cost of personalized services and therapies is a topic of heavy debate in health policy [70]. A major point of discussion is whether or not the absolute incremental increase in LVEF of up to 2.99%, infarct remodeling, and recovery of regional LV function translates into a meaningful clinical benefit at longer-term follow-up and justify the additional costs of cell-based interventions. There is currently a need for enhanced clinical evidence of the benefit of personalized medicine in order for marketed products to achieve coverage [70]. Stem cell therapies suffer from relative complexity and face significant cost constraints posed by the need to make the treatment affordable to large numbers of candidate patients. Moreover, the clinical requirement for a readily available treatment that can be prepared and administered in the majority of catheterization laboratories during the early phase of the disease remains a major challenge, especially of autologous cell therapies. To circumvent some of these obstacles, a rapid and relatively simple purification procedure should be proposed to isolate the most suitable cell population. In addition to safety and tracking, several logistical issues must also be addressed before stem cells can be used routinely in the clinic. While cell tracking methodologies allow researchers to determine migration patterns, the stem cells must target their desired destination and be retained there for a sufficient amount of time to achieve benefit. To facilitate targeting and enable clinical use, stem cells must be delivered easily and efficiently to their sites of application. Finally, the ease by which the cells can be obtained and the cost of cell preparation will also influence their transition to the clinic.
Conclusion and future outlook
Although we have witnessed remarkable progress in this exciting era of translational cell-based therapies for cardiovascular disorders, many outstanding questions remain. We have to realize that treatment responses to stem cells depends on interactions between the delivered stem cells and the cells of the host, making cell-based therapy far more complex, and host dependent than pharmacological therapy.
For the past decade, adult stem cells from the BM have been the most attractive cells for clinicians to be used in cell therapy because of their autologous nature and ease of isolation. Although the patients who received BM-cell transplant had a modest functional benefit, BM-cells did not reproducibly generate new myocardium. The detected benefit was most reasonably attributed to paracrine effects. Therefore beneficial effects might be based on inhibition or reversing of negative remodeling processes and the induction of neovascularization, which then inhibits apoptosis of cardiomyocytes especially within the border zone of the infarct. Thus the aim of regenerating cardiac muscle in humans has jet not been achieved. Despite identification of better candidate cell types than BM-cells and establishment of definitive protocols for stem cell propagation and differentiation, requirements to overcome include characterization of biomarkers to monitor stem cell fade after transplantation, validation of appropriate imaging modalities, model systems demonstrating interactions between stem cells and local environment and cell amplification procedures. Identification of molecules guiding differentiation processes by secretome analyses and bioinformatic approaches, will advance protein based therapies to promote healing and inhibit pathological remodeling of the heart after ischemia. On the side of the individual patient genomic and microenvironment profiles should be employed using genomic tools such as microarray analyses in a way to allow calculation of adverse interactions based on an individual's metabolism, and prediction of therapeutic outcomes [71]. None of the above obstacles will be overcome without concomitant efforts to develop critically needed enabling technologies.
Clearly, the field of stem cell therapy needs research that would serve towards the purpose of individualized treatments. The appearance of factors that influence disease prognosis and clinical outcome vary not only with donor stem cell function but also with genetic background of the individual host. Ultimately, personalization of health interventions, such as cell-based therapies, rests on a firm understanding of the mechanisms of person to person variations in treatment outcomes. Future studies to enhance the identification of molecular differences will allow a fundamental redefinition of diseases at the molecular and cellular levels and enable identification of patients with similar characteristics to estimate the outcome of treatment strategies [30, 72]. The heterogeneity among patient factors and the biology of different stem cell types merits the need for personalized approach to stem cell therapy and other cell-based treatments. Treatment employing stem cells holds promising potential as a therapy to regenerate damaged myocardium and might ultimately fulfill a large-scale of unmet clinical need to improve the quality of life for millions of people with ischemic heart disease.
The availability of pluripotent stem cells will however in the near future influence medicine in many other ways than tissue replacement. For instance, availability of cells that can generate many differentiated cell types, may lead to the development of protein or small molecule drugs that influence differentiation not only from pluripotent stem cells but also of multipotent stem cells residing in different tissues and this not only in vitro but also in vivo, much like the ability to culture hematopoietic stem cells led to the development of erythropoietin and granulocyte colony stimulating factor used in hematological disorders. The ability to generate cells with pluripotent characteristics that per definition differentiate in most cell types will also make it possible to generate in vitro models of human disease [73]. Moreover, availability of human cells differentiate to e.g. cardiomyocyte is already of great interest for the pharmaceutical industry for toxicity and metabolization studies.
References
Janssens S. Stem cells in the treatment of heart disease. Annu Rev Med. 2010;61:287–300.
Bates S. Progress towards personalized medicine. Drug Discov Today. 2010;15:115–20.
Ely S. Personalized medicine: individualized care of cancer patients. Transl Res. 2009;154:303–8.
Nelson TJ, Behfar A, Yamada S, Martinez-Fernandez A, Terzic A. Stem cell platforms for regenerative medicine. Clin Transl Sci. 2009;2:222–7.
Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–7.
Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9.
Urbich C, Rossig L, Dimmeler S. Restoration of cardiac function with progenitor cells. Novartis Found Symp. 2006;274:214–23. discussion 23–7, 72–6.
Wollert KC, Drexler H. Cell therapy for the treatment of coronary heart disease: a critical appraisal. Nat Rev Cardiol. 2010;7:204–15.
Meyer GP, Wollert KC, Drexler H. Stem cell therapy: a new perspective in the treatment of patients with acute myocardial infarction. Eur J Med Res. 2006;11:439–46.
Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44:1690–9.
Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI). Circulation. 2002;106:3009–17.
Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–32.
Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–5.
Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21.
Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–21.
Egeland T, Brinchmann JE. The REPAIR-AMI and ASTAMI trials: cell isolation procedures. Eur Heart J. 2007;28:2174–5. author reply 75.
Seeger FH, Tonn T, Krzossok N, Zeiher AM, Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–72.
Marenzi G, Bartorelli AL. Improved clinical outcome after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2007;28:2172–3. author reply 73–4.
Reffelmann T, Konemann S, Kloner RA. Promise of blood- and bone marrow-derived stem cell transplantation for functional cardiac repair: putting it in perspective with existing therapy. J Am Coll Cardiol. 2009;53:305–8.
Schachinger V, Assmus B, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, et al. Intracoronary infusion of bone marrow-derived mononuclear cells abrogates adverse left ventricular remodelling post-acute myocardial infarction: insights from the reinfusion of enriched progenitor cells and infarct remodelling in acute myocardial infarction (REPAIR-AMI) trial. Eur J Heart Fail. 2009;11:973–9.
Assmus B, Rolf A, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, et al. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail. 2010;3:89–96.
Dill T, Schachinger V, Rolf A, Mollmann S, Thiele H, Tillmanns H, et al. Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: results of the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. Am Heart J. 2009;157:541–7.
Wojakowski W, Kucia M, Kazmierski M, Ratajczak MZ, Tendera M. Circulating progenitor cells in stable coronary heart disease and acute coronary syndromes: relevant reparatory mechanism? Heart. 2008;94:27–33.
Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Wan W, Ratajczak J, Wojakowski W, et al. Hunt for pluripotent stem cell—regenerative medicine search for almighty cell. J Autoimmun. 2008;30:151–62.
Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208–16.
Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005;96:151–63.
Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer-Rasokat U, et al. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J Am Coll Cardiol. 2007;49:2341–9.
Spyridopoulos I, Hoffmann J, Aicher A, Brummendorf TH, Doerr HW, Zeiher AM, et al. Accelerated telomere shortening in leukocyte subpopulations of patients with coronary heart disease: role of cytomegalovirus seropositivity. Circulation. 2009;120:1364–72.
Spyridopoulos I, Erben Y, Brummendorf TH, Haendeler J, Dietz K, Seeger F, et al. Telomere gap between granulocytes and lymphocytes is a determinant for hematopoetic progenitor cell impairment in patients with previous myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:968–74.
Patel SA, King CC, Lim PK, Habiba U, Dave M, Porecha R, et al. Personalizing stem cell research and therapy: the arduous road ahead or missed opportunity? Curr Pharmacogenomics Person Med. 2010;8:25–36.
Pittenger MF. Mesenchymal stem cells from adult bone marrow. Methods Mol Biol. 2008;449:27–44.
Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20.
Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571–9.
Davani S, Marandin A, Mersin N, Royer B, Kantelip B, Herve P, et al. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation. 2003;108 Suppl 1:II253–8.
Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896–902.
Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19.
Vittet D, Prandini MH, Berthier R, Schweitzer A, Martin-Sisteron H, Uzan G, et al. Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood. 1996;88:3424–31.
Marchetti S, Gimond C, Iljin K, Bourcier C, Alitalo K, Pouyssegur J, et al. Endothelial cells genetically selected from differentiating mouse embryonic stem cells incorporate at sites of neovascularization in vivo. J Cell Sci. 2002;115:2075–85.
Min JY, Yang Y, Converso KL, Liu L, Huang Q, Morgan JP, et al. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J Appl Physiol. 2002;92:288–96.
Drukker M, Benvenisty N. The immunogenicity of human embryonic stem-derived cells. Trends Biotechnol. 2004;22:136–41.
Condic ML, Rao M. Regulatory issues for personalized pluripotent cells. Stem Cells. 2008;26:2753–8.
Klimanskaya I, Rosenthal N, Lanza R. Derive and conquer: sourcing and differentiating stem cells for therapeutic applications. Nat Rev Drug Discov. 2008;7:131–42.
Dowell JD, Rubart M, Pasumarthi KB, Soonpaa MH, Field LJ. Myocyte and myogenic stem cell transplantation in the heart. Cardiovasc Res. 2003;58:336–50.
Reinecke H, MacDonald GH, Hauschka SD, Murry CE. Electromechanical coupling between skeletal and cardiac muscle. Implications for infarct repair. J Cell Biol. 2000;149:731–40.
Leobon B, Garcin I, Menasche P, Vilquin JT, Audinat E, Charpak S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci USA. 2003;100:7808–11.
Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–83.
Siminiak T, Kalawski R, Fiszer D, Jerzykowska O, Rzezniczak J, Rozwadowska N, et al. Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: phase I clinical study with 12 months of follow-up. Am Heart J. 2004;148:531–7.
Dow J, Simkhovich BZ, Kedes L, Kloner RA. Washout of transplanted cells from the heart: a potential new hurdle for cell transplantation therapy. Cardiovasc Res. 2005;67:301–7.
Rosenstrauch D, Poglajen G, Zidar N, Gregoric ID. Stem celltherapy for ischemic heart failure. Tex Heart Inst J. 2005;32:339–47.
Becher MU, Nickenig G, Werner N. Regeneration of the vascular compartment. Herz. 2010;35:342–51.
Schuster MD, Kocher AA, Seki T, Martens TP, Xiang G, Homma S, et al. Myocardial neovascularization by bone marrow angioblasts results in cardiomyocyte regeneration. Am J Physiol Heart Circ Physiol. 2004;287:H525–32.
Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007.
Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9.
Yamanaka S, Takahashi K. Induction of pluripotent stem cells from mouse fibroblast cultures. Tanpakushitsu Kakusan Koso. 2006;51:2346–51.
Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1:39–49.
Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–41.
Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, et al. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–17.
Kuzmenkin A, Liang H, Xu G, Pfannkuche K, Eichhorn H, Fatima A, et al. Functional characterization of cardiomyocytes derived from murine induced pluripotent stem cells in vitro. FASEB J. 2009;23:4168–80.
Pfannkuche K, Liang H, Hannes T, Xi J, Fatima A, Nguemo F, et al. Cardiac myocytes derived from murine reprogrammed fibroblasts: intact hormonal regulation, cardiac ion channel expression and development of contractility. Cell Physiol Biochem. 2009;24:73–86.
Martinez-Fernandez A, Nelson TJ, Yamada S, Reyes S, Alekseev AE, Perez-Terzic C, et al. iPS programmed without c-MYC yield proficient cardiogenesis for functional heart chimerism. Circ Res. 2009;105:648–56.
Gersh BJ, Simari RD, Behfar A, Terzic CM, Terzic A. Cardiac cell repair therapy: a clinical perspective. Mayo Clin Proc. 2009;84:876–92.
Lian Q, Chow Y, Esteban MA, Pei D, Tse HF. Future perspective of induced pluripotent stem cells for diagnosis, drug screening and treatment of human diseases. Thromb Haemost. 2010;104:39–44.
Boyle AJ, Schulman SP, Hare JM, Oettgen P. Is stem cell therapy ready for patients? Stem cell therapy for cardiac repair. Ready for the next step. Circulation. 2006;114:339–52.
Chamuleau SA, Vrijsen KR, Rokosh DG, Tang XL, Piek JJ, Bolli R. Cell therapy for ischaemic heart disease: focus on the role of resident cardiac stem cells. Neth Heart J. 2009;17:199–207.
Torella D, Ellison GM, Mendez-Ferrer S, Ibanez B, Nadal-Ginard B. Resident human cardiac stem cells: role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2006;3 Suppl 1:S8–S13.
Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908.
Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21.
Rubart M, Field LJ. Stem cell differentiation: cardiac repair. Cells Tissues Organs. 2008;188:202–11.
Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–9.
Meckley LM, Neumann PJ. Personalized medicine: factors influencing reimbursement. Health Policy. 2010;94:91–100.
Kreiner T, Buck KT. Moving toward whole-genome analysis: a technology perspective. Am J Health Syst Pharm. 2005;62:296–305.
Arbab AS, Janic B, Haller J, Pawelczyk E, Liu W, Frank JA. In vivo cellular imaging for translational medical research. Curr Med Imag Rev. 2009;5:19–38.
Boheler KR. Pluripotency of human embryonic and induced pluripotent stem cells for cardiac and vascular regeneration. Thromb Haemost. 2010;104:23–9.
Acknowledgments
U. M. Becher and V. Tiyerili were supported by BONFOR (O-109.0028)
Disclosure
No potential conflicts of interest relevant to this article have been reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Becher, U.M., Tiyerili, V., Skowasch, D. et al. Personalized cardiac regeneration by stem cells–Hype or hope?. EPMA Journal 2, 119–130 (2011). https://doi.org/10.1007/s13167-011-0068-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13167-011-0068-z