Abstract
In wet peatlands, plant growth conditions are largely determined by local soil conditions, leading to locally adapted vegetation. Despite that Carex species are often the prevailing vascular plant species in fen peatlands of the temperate zone, information about how these species adapt to local environmental conditions is scarce. This holds true especially for below-ground plant traits and for adaptations to fen-typical nutrient level variations. To address this research gap, we investigated how different geographic origins (Germany, Poland, The Netherlands) of C. acutiformis and C. rostrata relate to their response to varying nutrient availability. We performed a common garden experiment with a controlled gradient of nutrient levels, and analyzed above- and below-ground biomass production of both Carex species from the different geographic origins. We related these traits to environmental conditions of the origins as characterized by vegetation composition-derived indicator values for ecological habitat conditions. While we detected high above-ground phenotypic plasticity of Carex from different origins, our data point to below-ground genotypic differences, potentially indicating local adaptation: Rhizome traits of C. rostrata differed significantly between origins with different nutrient indicator values. These results point towards differences in C. rostrata clonal spread behavior depending on local peatland conditions. Therefore, local adaptations of plant species and below-ground biomass traits should be taken into account when studying peatland vegetation ecology, as key functional traits can differ between genotypes within a single species depending on local conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The composition of mire vegetation is impacted foremost by the local environmental conditions, where water-logging poses special challenges for plants to establish and flourish. Therefore, mire vegetation composition starkly differs from the typical zonal vegetation and is considered being azonal, i.e., similar species compositions form despite large geographic distances between peatlands (Mucina et al. 2006; Sieben 2019). Still, local conditions, e.g., water level depth, mineral and nutrient concentrations, and pH, can vary widely across and even within peatlands (Bridgham et al. 1996; Rydin and Jeglum 2013; Wheeler and Proctor 2000). It remains unclear whether the same species genetically adapt to the local conditions, or whether they exhibit high phenotypic plasticity, i.e., whether the same genotype produces different phenotypes in response to locally different environmental conditions.
Ground- and surface water-fed peatlands (fens) in the temperate zone are often dominated by Carex L. species. This genus consists of over 2,000 species, many of which occur sympatrically (GLOBAL CAREX GROUP 2015). Closely related Carex species – which supposedly have similar ecological niches – tend to not occur at the same sites, whereas sympatric Carex species often occupy well-distinguishable niches (Waterway et al. 2016). At the same time, Carex populations of the same species, but from different geographic origins, show local adaptations, for example with regard to their growth and phosphate absorption rates and, more generally, with regard to their realized ecological niches, and can also exhibit morphological differences (Chapin and Chapin 1981; Chapin III and Oechel, 1983; Choler and Michalet 2002; Hájková et al. 2008; Więcław et al. 2021). While some studies about local adaptations of Carex species are thus available, we lack information about intra-specific adaptations of this fen-dominating genus to differences in nutrient availability. Nutrient levels can vary considerably within and between peatlands (Bridgham et al. 1996; Navrátilová et al. 2017; Rydin and Jeglum 2013; Wassen et al. 1990, 2002; Wheeler and Proctor 2000). Inter-specific differences in the response of sedges to increasing nutrient levels are rather well understood and include species-specific increased biomass production and shoot:root ratios (Aerts et al. 1992; Hinzke et al. 2021; Pérez-Corona and Verhoeven 1996; Veerkamp et al. 1980), but how the same Carex species adapts to locally varying nutrient levels is as yet unknown.
To address this research gap, we performed a common garden mesocosm experiment to detect genetic differentiation, which could indicate local genetic adaptations and phenotypic plasticity to variations in nutrient levels of two common Carex species, both sampled from different geographic origins (Germany, Poland, The Netherlands). For our study, we chose two Carex species naturally adapted to different nutrient levels: the lesser pond-sedge C. acutiformis Ehrh. and the beaked sedge C. rostrata Stokes ex With. Both grow rhizomatous (Bernard 1990; Konings et al. 1992) and are common fen species throughout temperate and southern boreal Europe (Egorova 1999; Landucci et al. 2020; Mossberg and Stenberg 2003; Peterka et al. 2017).
We analyzed plant above- and below-ground biomass production, and determined biomass nutrient contents, as a measure for plant performance under different nutrient levels. We also performed vegetation surveys, based on which we calculated ecological indicator values for habitat conditions at the sampling sites of the Carex specimens used in this study, to relate reactions to nutrient level variations to conditions at the site of origin. We hypothesized (i) genetic adaptation of fen sedges with regard to nutrient levels. That is, we expected that plants from sites exhibiting high indicator values for nutrients would produce more biomass at higher nutrient levels, whereas those stemming from sites with lower indicator values for nutrients would show less biomass increase or even decrease with increasing nutrient levels. Additionally, we assumed that (ii) Carex species typically associated with different nutrient levels would show different reaction norms to variations in nutrient levels.
Methods
Sampling of Carex Specimens from Different Geographic Origins
For each of the two species C. acutiformis and C. rostrata we sampled specimens in November 2017 from three geographic origins (Table 1, see also Hinzke et al. 2021). We sampled in three to four different locations per geographic origin to collect different individuals rather than a single clone. For simplicity, we will refer to the geographic origins in the following by the names of the countries (Germany, Poland, The Netherlands). We chose these three geographic origins as they are sufficiently far away from each other to theoretically allow for site-specific genetic differentiation. In addition, they represent a gradient of nutrient status (mostly eu- to hypertrophic in The Netherlands and Germany, mesotrophic in Poland; Cabezas et al. 2014; Koch and Jurasinski 2015; Liu et al. 2021; Wassen and Olde Venterink, 2006), and different drainage histories (largely long-term drainage with partial rewetting in The Netherlands and Germany, mostly undrained in Poland). Drainage itself leads to increased peat mineralization and nitrogen release (Olde Venterink et al. 2009), and rewetting often to higher phosphorous mobilization (Emsens et al. 2017; Zak et al. 2010). Plants, consisting of one shoot and a few roots and rhizomes, were kept in small peat-filled pots in a common sand bed over winter.
Estimation of Ecological Habitat Conditions Based on Vegetation Indicator Values
Our characterization of ecological habitat conditions of the Carex sampling sites was based on site-specific vegetation. We performed vegetation surveys at the sampling locations of both Carex species, and assigned the plant species ecological indicator values for ecological habitat conditions as given by Ellenberg indicator values. Ellenberg indicator values assign numeric values (1–9) to species-specific optima estimates observed along gradients of environmental conditions (Ellenberg et al. 2001). This expert system of vegetation-derived indicator values has the important advantage to represent long-term conditions, rather than a snapshot of highly dynamic environmental parameters (Bartelheimer and Poschlod 2016; Schaffers and Sýkora 2000). To give an example for our study, C. acutiformis has an Ellenberg indicative value for nutrients (EllN) of 5, whereas C. rostrata has an EllN of 3 (EllN according to GermanSL, Jansen and Dengler 2008; see below). This means that C. acutiformis prefers more nutrient-rich soils than C. rostrata.
Vegetation surveys of 4 m2 plots including both vascular plants and mosses were conducted in June/July 2017 in Poland (2 relevés for C. acutiformis, 9 for C. rostrata sampling locations) and 2021 in Germany (4 relevés for C. acutiformis, 2 for C. rostrata sampling locations) and The Netherlands (3 relevés each for C. acutiformis and C. rostrata) on the sampling sites of the Carex specimens used in this study. Site-specific ecological indicator values were then calculated using the Ellenberg indicator values as given in the “ecodbase” database of the “GermanSL v. 1.5”, which contains taxonomic reference lists for vegetation databases in Germany (Jansen and Dengler 2008), and using novel indicator values for nutrients for bryophytes (Simmel et al. 2021). We weighted the indicator values by the respective species’ coverage to generate cover-weighted indicator values. “Indifferent” assignments were not used for indicator value calculation. For entries in the ecodbase database marked as “doubtful” (i.e., indicator value with question mark), the indicator value given was used. In order to assess representativeness of the indicator value calculation, we also calculated the coverage of plants with a numeric indicator value relative to the total coverage per plot.
Mesocosm Experiments
Our study was conducted in the course of a 12-week mesocosm common garden experiment from May to September 2018 (Hinzke et al. 2021). A nutrient level gradient with 12 steps was created via fertilization combined with watering, with each mesocosm corresponding to one nutrient level (Supp. Table 1), resulting in a total of 24 mesocosms. In each mesocosm (a 125 l pot filled with peat), nine specimens of the same species and geographic origin were planted. Pots were fertilized every two weeks to adjust the specific nutrient level per pot. Additionally, pots were watered twice a week initially and then daily. Each pot was connected via a tube to one canister, and water from that canister was used to water the same pot again. This way, the water level was kept within a few centimeters below the peat surface and nutrients stayed in the same mesocosm. At the typical end of the below-ground growing season in temperate ecosystems (i.e., September; Schwieger et al. 2019), plants were harvested, separated into below- and above-ground parts, dried, and weighed, rhizomes were counted and their length was measured. Shoot C and N contents, as well as shoot and root P and K contents were determined as described in Hinzke et al. (2021).
Statistical Evaluation of Plant Biomass Characteristics
Data evaluation for Carex biomass characteristics was based on the best models identified in a former study (Hinzke et al. 2021). In brief, we fitted total, shoot, root, and rhizome biomass production using a Gompertz growth model. Likewise, we used a Gompertz growth model to model data of total rhizome lengths and total rhizome numbers. For the ratio of above- to below-ground biomass, we used a logistic growth model. To model specific rhizome length (total rhizome length divided by total rhizome weight) and shoot C:N ratios, we employed linear models (see Hinzke et al. (2021) for C:N ratio) and compared model performance with predictors “total N” only, “geographic origin” only, and combining both, using ANOVA (Supp. Table 2). Additionally, we modelled %P and %K in root and shoot biomass with linear models, and %N in shoot biomass using a logistic growth model. These models were selected based on model evaluation with diagnostic plots. Non-overlap of 95% confidence bands was interpreted as significant difference. Analyses were done in R 4.0.5 (R Core Team, 2021), using for data reformatting the packages dplyr 1.0.5 (Wickham et al. 2021a), purrr_0.3.4 (Henry and Wickham 2020), and tidyr_1.1.3 (Wickham 2021), for statistical analysis the packages AICcmodavg 2.3-1 (Mazerolle 2020), boot_1.3–27 (Canty and Ripley 2021), broom_0.7.6 (Robinson et al. 2021), nlstools_1.0–2 (Baty et al. 2015), and for graphics generation and export egg_0.4.5 (Auguie 2019), ggnewscale 0.4.5 (Campitelli 2021), ggplot2_3.3.3 (Wickham 2016), and svglite_2.0.0 (Wickham et al. 2021b).
Results
Carex Sites of Origin Differ in Ecological Indicator Values for Nutrients
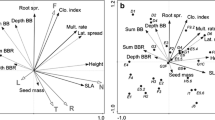
Ecological indicator values for site conditions calculated from vegetation surveys of C. acutiformis and C. rostrata sampling locations were mostly similar for the same species between geographic origins, with the notable exceptions of nutrient and temperature values for C. rostrata, and of moisture values for C. acutiformis (Fig. 1). Cover-weighted nutrient and temperature values of C. rostrata sampling locations in Poland were often lower than those for the other two origins. With regard to moisture values for C. acutiformis sampling locations, these were highest for Poland. Overall, differences between sampling locations of the two different species were more pronounced. Except for soil reaction values (pH), the relative coverage of plants with “indifferent” indicator value assignment was always below 11% (Supp. Table 3).
Ecological indicator values for moisture, light, nutrients, pH, and temperature of sampling locations of Carex acutiformis and C. rostrata. Indicator values were weighted by species coverage. Dots: Indicator values of individual plots, colored by geographic origin. Saturation of dots: coverage of plants which had a numeric indicator value assigned relative to total plot vegetation coverage. Boxplots: summary statistics of the indicator value for sampling locations of C. acutiformis or C. rostrata
Biomass Production in Carex Depends on Nutrient Level and Geographic Origin
Carex from different geographic origins generally did not differ significantly in their overall and organ-specific biomass production (Fig. 2). However, root biomass production and number of rhizomes at intermediate to high nutrient levels were lower for C. acutiformis from The Netherlands as compared to the other origins. Moreover, for Polish C. rostrata, number of rhizomes and total rhizome lengths were significantly lower than were those of the other origins. At the highest nutrient level, C. rostrata from Poland had a total of 512 rhizomes with a total rhizome length of 91 m, whereas C. rostrata from Germany had 556 rhizomes (104 m) and C. rostrata from The Netherlands 709 rhizomes (124 m). At intermediate nutrient levels, Polish C. rostrata had 150 to over 200 rhizomes less than those from Germany and The Netherlands, corresponding to a total length difference of 24 to 37 m (Supp. Table 4). Concomitantly, specific rhizome length of C. rostrata differed significantly between origins, with the lowest specific rhizome length observed for Polish C. rostrata. In addition, C. rostrata specific rhizome length showed a significant interaction between origins and nutrient levels, with specific rhizome length decreasing stronger across nutrient levels for C. rostrata from Poland and Germany as compared to those from The Netherlands. Length of individual rhizomes did not show a clear pattern or difference between either nutrient levels or origins, aside from C. acutiformis from The Netherlands producing a somewhat higher number of long rhizomes than those from the other origins (Supp. Figure 1).
In addition, the above-/below-ground biomass ratio of C. acutiformis from The Netherlands was slightly higher at intermittent nutrient levels than those of the other origins. Above-/below-ground biomass ratio also appeared to be intermittently higher for C. rostrata with Polish origin than for those of the other origins, but this difference was driven by only one data point.
While for C. acutiformis no significant difference in shoot C:N ratios between origins was found, shoot C:N ratio of C. rostrata from The Netherlands was significantly lower, and, correspondingly, %N in shoot was higher, than were those of the other origins (Supp. Figure 2, Supp. Table 2).
Specimens from different origins did not differ significantly in their %P or %K in root and shoot biomass across the nutrient gradient, apart from %P in C. rostrata roots from Poland being higher than in the other origins at the highest nutrient level (Supp. Figure 3).
Total biomass production, as well as biomass production of shoots, roots, and rhizomes, ratio of above- to below-ground biomass, number of rhizomes, total rhizome length, and specific rhizome length of (A) Carex acutiformis and (B) C. rostrata, sampled from different geographic origins, in mesocosms. Total N [g]: total nitrogen per mesocosm. Dots: Raw values, lines: regression curves, semitransparent ribbons: 95% confidence bands
Maximum differences of plant biomass metrics of the same species from different origins occurred mostly at very high to highest nutrient levels for C. acutiformis, but also at intermediate nutrient levels for C. rostrata (Fig. 2, Supp. Table 4). For C. acutiformis, maximum differences always occured between Germany and the other origins, and for C. rostrata, between Poland and the other origins. Differences between C. acutiformis and C. rostrata from the same origin were almost always highest at intermediate to high nutrient values. Maximum within-species differences for biomass traits were mostly about half to two thirds as high as between-species differences.
Discussion
Our study indicates genetic differentiation of Carex species due to geographically differing environmental conditions in fen peatlands, in accordance with our hypothesis (i): we found significant differences in plant biomass production of Carex from different origins in our common garden experiment. This genetic differentiation hints towards local genetic adaptations. At the same time, both Carex species from all origins exhibited high phenotypic plasticity. In contrast to our hypothesis (ii), both species showed similar total biomass production profiles across the whole nutrient gradient, despite their differences in natural nutrient availability preferences.
Genetic differentiation of Carex from different origins was suggested by notably fewer rhizomes with a lower total length and lower specific rhizome length in C. rostrata from Poland as compared to the other origins. Simultaneously, the Polish sampling sites for this species had mostly lower indicator values for nutrients than the sampling sites in Germany and The Netherlands. At the same time, the differences in C. acutiformis root biomass and number of rhizomes between origins do not seem to be correlated to site-specific ecological indicator values. In the following, we propose two, potentially synergistic, explanations for the origin-specific differences observed in C. rostrata rhizome growth.
-
(A)
C. rostrata from different origins differ in their clonal spread or foraging behavior, with more efficient local resource use – i.e., less length increase per unit biomass investment – in Poland and a more explorative rhizome growth in the other origins. Causes for origin-specific clonal spread differences might be higher competition pressure or pressure of other stressors at the Polish than at the other two sites, or a lack of ressources at the other sites. Such patterns of differential clonal growth behavior depending on local environmental conditions also occur in the sedges Scirpus planiculmis (Ning et al. 2014), Scirpus olneyi (Ikegami et al. 2007) and Carex brevicuspis (Deng et al. 2013).
-
(B)
Gas exchange efficiency of C. rostrata differs between sites, potentially due to site-specific differences in long-term drainage history. While the Ellenberg indicator values for moisture did not differ between origins of C. rostrata, sampling sites in Poland were mostly undrained, in contrast to the long-term drained sites in Germany and The Netherlands (see Methods). Potentially thicker rhizomes (i.e., same total biomass, but more aerenchymes) in the Polish C. rostrata might allow for a more efficient gas exchange in water-logged soils (Sharma et al. 2008). Similarly, in the sedge Scirpus planiculmis rhizome, its morphology, but not biomass, is soil moisture-dependent (Ning et al. 2014). In Germany and The Netherlands, a lower specific rhizome length (i.e., longer rhizome per weight unit) might have also aided re-colonization after rewetting.
The differences in rhizomatous growth are probably not related to nutrient storage per se, as total rhizome weight and thus total investment into storage tissues did not differ between C. rostrata of all three origins. However, the lower shoot C:N ratio and higher %N in shoots of C. rostrata from The Netherlands, where sampling locations were associated with high nutrient indicator values, might point to local variations in nutrient uptake efficiency.
High phenotypic plasticity was visible in almost all measured traits in both species. Phenotypic plasticity enables plants to deal with changing environmental conditions (Valladares et al. 2007). Phenotypic plasticity might therefore be especially advantageous in heterogeneous habitats, such as peatlands. More generally, considerable phenotypic plasticity occurs in different Carex species (Więcław 2017; Więcław et al. 2021, 2022), including alpine Carex species, i.e., in species which also live in rather heterogeneous habitats (Schmidt et al. 2018). An advantage of increased biomass at higher nutrient levels, as observed in our study, could be higher ability to deal with competition, e.g., for light (Kotowski et al. 2006). In accordance with this, boreal peatland vascular plants exhibit a plastic response to N addition, with plant height being especially important at higher N levels (Lin et al. 2020). A notable exception from the general pattern of high phenotypic plasticity was the specific rhizome length, where C. acutiformis showed no response to increasing nutrient levels, whereas C. rostrata specific rhizome length decreased. In general, rhizome morphology responses to nutrients and competition are highly species-specific (reviewed in de Kroon and Hutchings 1995; Hutchings and de Kroon 1994). The reasons for this lack of phenotypic plasticity, and whether it is due to plasticity costs outweighing advantages, or whether it might even impact the performance of C. acutiformis, warrant further research.
Overall, maximum biomass trait differences of Carex for the same species and nutrient level encompassed of up to two thirds of that between species in our study. This means that specimens of the same Carex species can behave almost as different as two different species. Such high intra-specific reaction magnitudes impact how plant species respond to environmental changes (see also Malyshev et al. 2016; Münzbergová et al. 2017). Therefore, a species’ reaction magnitude to environmental changes needs to be taken into account for example for restoration measures involving re-introduction or assisted colonization of plants: For choosing suitable species, not only a species’ general ecological preferences are important, but also local adaptations of specific populations (van Andel 1998), and the ability of these populations to deal with current and future environmental changes.
It remains to be elucidated whether the intra-specific differences in Carex from different origins have an adaptive value. Intra-specific, genetically fixed clonal growth differences might lead to niche partitioning, as proposed for clonal growth differences between Carex species (Tammaru et al. 2021). This could then lead to formation of ecotypes, i.e., genetically fixed geographic varieties, which occur in other Carex species (Chapin and Chapin 1981; Chapin III and Oechel, 1983; Choler and Michalet 2002; Hájková et al. 2008). Intriguingly, root anatomical traits of Carex can be used to predict their Ellenberg indicator values for different ecological gradients (Hoffmann et al. 2021). It would be interesting to see whether there is a similar relationship between rhizome traits and ecological optima, and whether such a relationship can also be found on the level of intra-specific variation.
In summary, we found indications for below-ground genetic adaptation and high phenotypic plasticity above- and below-ground for two common and widely distributed Carex species of temperate fen peatlands. The data presented here underline that within-species differences in trait responses to environmental gradients can be in the same order of magnitude as those between species, and that these differences might not be visible when only taking into account the more easily accessible above-ground biomass traits. Our study prompts further research into local intra-specific adaptations of Carex species with regard to clonal spread and nutrient uptake, e.g., considering rhizome C:N ratios, as well as into below-ground biomass traits, in particular of potentially peat-forming plant species.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Aerts R, de Caluwe H, Konings H (1992) Seasonal allocation of biomass and nitrogen in four Carex species from mesotrophic and eutrophic fens as affected by nitrogen supply. J Ecol 80:653–664. doi:https://doi.org/10.2307/2260857
Auguie B (2019) egg: extensions for “ggplot2”: custom geom, custom themes, plot alignment, labelled panels, symmetric scales, and fixed panel size. R package version 0.4.5. Available at: https://cran.r-project.org/package=egg
Bartelheimer M, Poschlod P (2016) Functional characterizations of Ellenberg indicator values - a review on ecophysiological determinants. Funct Ecol 30:506–516. doi:https://doi.org/10.1111/1365-2435.12531
Baty F, Ritz C, Charles S, Brutsche M, Flandrois J-P, Delignette-Muller M-L (2015) A toolbox for nonlinear regression in R: The package nlstools. J Stat Softw 66:1–21. doi:https://doi.org/10.18637/jss.v066.i05
Bernard JM (1990) Life history and vegetative reproduction in Carex. Can J Bot 68:1441–1448. doi:https://doi.org/10.1139/b90-182
Bridgham SD, Pastor J, Janssens JA, Chapin C, Malterer TJ (1996) Multiple limiting gradients in peatlands: a call for a new paradign. https://doi.org/10.1007/BF03160645
Cabezas A, Pallasch M, Schönfelder I, Gelbrecht J, Zak D (2014) Carbon, nitrogen, and phosphorus accumulation in novel ecosystems: Shallow lakes in degraded fen areas. Ecol Eng 66:63–71. doi:https://doi.org/10.1016/j.ecoleng.2013.10.037
Campitelli E (2021) ggnewscale: Multiple Fill and Colour Scales in “ggplot2”. R package version 0.4.5. Available at: https://cran.r-project.org/package=ggnewscale
Canty A, Ripley B (2021) boot: Bootstrap R (S-Plus) Functions. R package version 1.3–27
Chapin III, Chapin MC (1981) Ecotypic differentiation of growth processes in Carex aquatilis along latitudinal and local gradients. Ecology 62:1000–1009
Chapin III, Oechel WC (1983) Photosynthesis, respiration, and phosphate absorption by Carex aquatilis ecotypes along latitudinal and local environmental gradients. Ecology 64:743–751
Choler P, Michalet R (2002) Niche differentiation and distribution of Carex curvula along a bioclimatic gradient in the southwestern Alps. J Veg Sci 13:851–858. doi:https://doi.org/10.1111/j.1654-1103.2002.tb02114.x
de Kroon H, Hutchings MJ (1995) Morphological plasticity in clonal plants: the foraging concept reconsidered. J Ecol 83:143–152
Deng Z-M, Chen X-C, Xie Y-H, Pan Y, Li F, Hou Z-Y et al (2013) Plasticity of the clonal growth in the wetland sedge Carex brevicuspis along a small-scale elevation gradient in Dongting Lake Wetlands, China. Ann Bot Fenn 50:151–159. doi:https://doi.org/10.5735/085.050.0305
Egorova TV (1999) The sedges (Carex L.) of Russia and adjacent states (within the limits of the former USSR). St. Petersburg: Russian Academy of Sciences, Komarov Botanical Institute
Ellenberg H, Weber HE, Düll R, Wirth V, Werner W (2001) Zeigerwerte von Pflanzen in Mitteleuropa, 3rd edn. Erlich Goltze GmbH & Co KG, Göttingen
Emsens WJ, Aggenbach CJS, Smolders AJP, Zak D, van Diggelen R (2017) Restoration of endangered fen communities: the ambiguity of iron–phosphorus binding and phosphorus limitation. J Appl Ecol 54:1755–1764. doi:https://doi.org/10.1111/1365-2664.12915
GLOBAL CAREX GROUP (2015) Making Carex monophyletic (Cyperaceae, tribe Cariceae): a new broader circumscription. Bot J Linn Soc 179:1–42
Hájková P, Hájek M, Apostolova I, Zelený D, Dítě D (2008) Shifts in the ecological behaviour of plant species between two distant regions: Evidence from the base richness gradient in mires. J Biogeogr 35:282–294. doi:https://doi.org/10.1111/j.1365-2699.2007.01793.x
Henry L, Wickham H (2020) purrr: Functional Programming Tools. R package version 0.3.4. Available at: https://cran.r-project.org/package=purrr
Hinzke T, Li G, Tanneberger F, Seeber E, Aggenbach C, Lange J et al (2021) Potentially peat-forming biomass of fen sedges increases with increasing nutrient levels. Funct Ecol 1579–1595. doi:https://doi.org/10.1111/1365-2435.13803
Hoffmann MH, Gebauer S, Lühmann R (2021) Root anatomy predicts ecological optima in Carex (Cyperaceae) in terms of Ellenberg indicator values. Ecol Indic 129:107979. doi:https://doi.org/10.1016/j.ecolind.2021.107979
Hutchings MJ, de Kroon H (1994) Foraging in plants: the role of morphological plasticity in resource acquisition. Adv Ecol Res 25:159–238. doi:https://doi.org/10.1016/S0065-2504(08)60215-9
Ikegami M, Whigham DF, Werger MJA (2007) Responses of rhizome length and ramet production to resource availability in the clonal sedge Scirpus olneyi A. Gray. Plant Ecol 189:247–259. doi:https://doi.org/10.1007/s11258-006-9181-9
Jansen F, Dengler J (2008) GermanSL - Eine universelle taxonomische Referenzliste für Vegetationsdatenbanken in Deutschland. Tuexenia 28:239–253
Koch M, Jurasinski G (2015) Four decades of vegetation development in a percolation mire complex following intensive drainage and abandonment. Plant Ecol Divers 8:49–60. doi:https://doi.org/10.1080/17550874.2013.862752
Konings H, Verhoeven JTA, de Groot R (1992) Growth characteristics and seasonal allocation patterns of biomass and nutrients in Carex species growing in floating fens. Plant Soil 147:183–196
Kotowski W, Thörig W, van Diggelen R, Wassen MJ (2006) Competition as a factor structuring species zonation in riparian fens - a transplantation experiment. Appl Veg Sci 9:231–240. doi:https://doi.org/10.1111/j.1654-109x.2006.tb00672.x
Landucci F, Šumberová K, Tichý L, Hennekens S, Aunina L, Biță-Nicolae C et al (2020) Classification of the European marsh vegetation (Phragmito-Magnocaricetea) to the association level. Appl Veg Sci 23:297–316. doi:https://doi.org/10.1111/avsc.12484
Lin G, Zeng DH, Mao R (2020) Traits and their plasticity determine responses of plant performance and community functional property to nitrogen enrichment in a boreal peatland. Plant Soil 449:151–167. doi:https://doi.org/10.1007/s11104-020-04478-4
Liu W, Fritz C, Nonhebel S, Everts HF, Grootjans AP (2021) Landscape-level vegetation conversion and biodiversity improvement after 33 years of restoration management in the Drentsche Aa brook valley. Restor Ecol e13601. doi:https://doi.org/10.1111/rec.13601
Malyshev AV, Arfin Khan MAS, Beierkuhnlein C, Steinbauer MJ, Henry HAL, Jentsch A et al (2016) Plant responses to climatic extremes: Within-species variation equals among-species variation. Glob Chang Biol 22:449–464. doi:https://doi.org/10.1111/gcb.13114
Mazerolle MJ (2020) AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). R package version 2.3-1. Available at: https://cran.r-project.org/package=AICcmodavg
Mossberg B, Stenberg L (2003) Den nya nordiska floran. Wahlström & Widstrand, Stockholm
Mucina L, Rutherford MC, Powrie LW (2006) Inland azonal vegetation. Strelitzia 19:616–657
Münzbergová Z, Hadincová V, Skálová H, Vandvik V (2017) Genetic differentiation and plasticity interact along temperature and precipitation gradients to determine plant performance under climate change. J Ecol 105:1358–1373. doi:https://doi.org/10.1111/1365-2745.12762
Navrátilová J, Hájek M, Navrátil J, Hájková P, Frazier RJ (2017) Convergence and impoverishment of fen communities in a eutrophicated agricultural landscape of the Czech Republic. Appl Veg Sci 20:225–235. doi:https://doi.org/10.1111/avsc.12298
Ning Y, Zhang ZX, Cui LJ, Zou CL (2014) Adaptive significance of and factors affecting plasticity of biomass allocation and rhizome morphology: a Case study of the clonal plant Scirpus planiculmis (Cyperaceae). Pol J Ecol 62:77–88. doi:https://doi.org/10.3161/104.062.0108
Olde Venterink H, Kardel I, Kotowski W, Peeters W, Wassen MJ (2009) Long-term effects of drainage and hay-removal on nutrient dynamics and limitation in the Biebrza mires, Poland. Biogeochemistry 93, 235–252. doi:https://doi.org/10.1007/s10533-009-9300-5
Pérez-Corona ME, Verhoeven JTA (1996) Effects of soil P status on growth and P and N uptake of Carex species from fens differing in P-availability. Acta Bot Neerl 45:381–392. doi:https://doi.org/10.1111/j.1438-8677.1996.tb00524.x
Peterka T, Hájek M, Jiroušek M, Jiménez-Alfaro B, Aunina L, Bergamini A et al (2017) Formalized classification of European fen vegetation at the alliance level. Appl Veg Sci 20:124–142. doi:https://doi.org/10.1111/avsc.12271
R Core Team (2021) R: A language and environment for statistical computing. Available at: https://www.r-project.org/
Robinson D, Hayes A, Couch S (2021) broom: convert statistical objects into tidy tibbles. R package version 0.7.6. Available at: https://cran.r-project.org/package=broom
Rydin H, Jeglum JK (2013) The Biology of Peatlands, 2nd edn. Oxford University Press, Oxford
Schaffers AP, Sýkora KV (2000) Reliability of Ellenberg indicator values for moisture, nitrogen and soil reaction: a comparison with field measurements. J Veg Sci 11:225–244. doi:https://doi.org/10.2307/3236802
Schmidt L, Schmid B, Oja T, Fischer M (2018) Genetic differentiation, phenotypic plasticity and adaptation in a hybridizing pair of a more common and a less common Carex species. Alp Bot 128:149–167. doi:https://doi.org/10.1007/s00035-018-0211-8
Schwieger S, Blume-Werry G, Peters B, Smiljanić M, Kreyling J (2019) Patterns and drivers in spring and autumn phenology differ above- and belowground in four ecosystems under the same macroclimatic conditions. Plant Soil 445:217–229. doi:https://doi.org/10.1007/s11104-019-04300-w
Sharma P, Asaeda T, Fujino T (2008) Effect of water depth on the rhizome dynamics of Typha angustifolia. Wetl Ecol Manag 16:43–49. doi:https://doi.org/10.1007/s11273-007-9055-5
Sieben EJJ (2019) Zonal and azonal vegetation revisited: How is wetland vegetation distributed across different zonobiomes. Austral Ecol 44:449–460. doi:https://doi.org/10.1111/aec.12679
Simmel J, Ahrens M, Poschlod P (2021) Ellenberg N values of bryophytes in Central Europe. J Veg Sci 32:e12957. doi:https://doi.org/10.1111/jvs.12957
Tammaru K, Košnar J, Abbas AF, Barta KA, de Bello F, Harrison S et al (2021) Ecological differentiation of Carex species coexisting in a wet meadow: Comparison of pot and field experiments. Acta Oecol 110:103692. doi:https://doi.org/10.1016/j.actao.2020.103692
Valladares F, Gianoli E, Gómez JM (2007) Ecological limits to plant phenotypic plasticity. New Phytol 176:749–763. doi:https://doi.org/10.1111/j.1469-8137.2007.02275.x
van Andel J (1998) Intraspecific variability in the context of ecological restoration projects. Perspect Plant Ecol Evol Syst 1/2:221–237. doi:https://doi.org/10.1078/1433-8319-00060
Veerkamp MT, Corré WJ, Atwell BJ, Kuiper PJC (1980) Growth rate and phosphate utilization of some Carex species from a range of oligotrophic to eutrophic swamp habitats. Physiol Plant 50:237–240. doi:https://doi.org/10.1111/j.1399-3054.1980.tb04456.x
Wassen MJ, Barendregt A, Palczynski A, de Smidt JT, de Mars H (1990) The relationship between fen vegetation gradients, groundwater flow and flooding in an undrained valley mire at Biebrza, Poland. J Ecol 78:1106–1122
Wassen MJ, Venterink O, H (2006) Comparison of nitrogen and phosphorus fluxes in some European fens and floodplains. Appl Veg Sci 9:213–222. doi:https://doi.org/10.1111/j.1654-109x.2006.tb00670.x
Wassen MJ, Peeters WHM, Venterink O, H (2002) Patterns in vegetation, hydrology, and nutrient availability in an undisturbed river floodplain in Poland. Plant Ecol 165:27–43. doi:https://doi.org/10.1023/A:1021493327180
Waterway MJ, Martins KT, Dabros A, Prado A, Lechowicz MJ (2016) Ecological and evolutionary diversification within the genus Carex (Cyperaceae): Consequences for community assembly in subarctic fens. Syst Bot 41:558–579. doi:https://doi.org/10.1600/036364416X692514
Wheeler BD, Proctor MCF (2000) Ecological gradients, subdivisions and terminology of north-west European mires. J Ecol 88:187–203. doi:https://doi.org/10.1046/j.1365-2745.2000.00455.x
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis, 2nd edn. Springer International Publishing, New York
Wickham H (2021) tidyr: tidy messy data. R package version 1.1.3. Available at: https://cran.r-project.org/package=tidyr
Wickham H, François R, Henry L, Müller K (2021a) dplyr: a grammar of data manipulation. R package version 1.0.5. Available at: https://cran.r-project.org/package=dplyr
Wickham H, Henry L, Pedersen TL, Luciani TJ, Decorde M, Lise V(2021b) svglite: an “SVG” graphics device. R package version 2.0.0. Available at: https://cran.r-project.org/package=svglite
Więcław H (2017) Within-species variation among populations of the Carex flava complex as a function of habitat conditions. Plant Ecol Divers 10:443–451. doi:https://doi.org/10.1080/17550874.2018.1440442
Więcław H, Bosiacka B, Hrivnák R, Dajdok Z, Mesterházy A, Koopman J (2022) Morphological variability of Carex buekii (Cyperaceae) as a function of soil conditions: a case study of the Central European populations. Sci Rep 12:11761. doi:https://doi.org/10.1038/s41598-022-15894-0
Więcław H, Szenejko M, Kull T, Sotek Z, Rębacz-Maron E, Koopman J (2021) Morphological variability and genetic diversity in Carex buxbaumii and Carex hartmaniorum (Cyperaceae) populations. PeerJ 9, e11372. doi:https://doi.org/10.7717/peerj.11372
Zak D, Wagner C, Payer B, Augustin J, Gelbrecht J (2010) Phosphorus mobilization in rewetted fens: the effect of altered peat properties and implications for their restoration. Ecol Appl 20:1336–1349. doi:https://doi.org/10.1890/08-2053.1
Acknowledgements
We acknowledge funding of the project REPEAT by BiodivERsA within the Horizon 2020 ERA-NET COFUND scheme (DFG JO 332/15 − 1, BELSPO BR/175/A1, NCN 2016/22/Z/NZ8/00001) and of the project DESIRE by the EU Interreg Baltic Sea Programme 2014–2020 (Index No.: R3.071, Project No.: #R091), which is a flagship project of the EU Strategy for the Baltic Sea Region in the Policy Area “Nutri”. It is financed by the European Regional Development Fund (ERDF), the European Neighbourhood Instrument (ENI), and the Russian national funding.
We thank Ilka Beil, Gesche Blume-Werry, Sascha Bock, Hans Joosten, and Laurenz Teuber for their input to the experimental design and implementation. Manuel Ewen helped with XRF analyses at the laboratory of the Institute of Landscape Ecology at University of Muenster. Reference materials for XRF analyses were kindly provided by Harald Biester (TU Braunschweig) and Johan Rydberg (University of Umea). We would like to thank all who provided help during the mesocosm experiment and all members of the REPEAT team.
Funding
We acknowledge funding of the project REPEAT by BiodivERsA within the Horizon 2020 ERA-NET COFUND scheme (DFG JO 332/15 − 1, BELSPO BR/175/A1, NCN 2016/22/Z/NZ8/00001) and of the project DESIRE by the EU Interreg Baltic Sea Programme 2014–2020 (Index No.: R3.071, Project No.: #R091), which is a flagship project of the EU Strategy for the Baltic Sea Region in the Policy Area “Nutri”. It is financed by the European Regional Development Fund (ERDF), the European Neighbourhood Instrument (ENI), and the Russian national funding.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
ES, FT, JK, and WK designed and coordinated experiments; CA and ŁK contributed to the experimental design; collection of the sedges and vegetation relevés were conducted by CA, DM, ES as well as ŁK and EP in The Netherlands, Germany and Poland, respectively; SD, GL, ES, FT, EP, JK, WK ran the experiment and sampled the data; TH analyzed the data and wrote the manuscript with input from all authors; JL coordinated C:N and XRF analyses of biomass samples; K-HK provided XRF analyses of biomass samples; MB and MS performed specific literature research and were involved in data interpretation and discussion. All authors gave input and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
All authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hinzke, T., Tanneberger, F., Aggenbach, C. et al. Response Patterns of Fen Sedges to a Nutrient Gradient Indicate both Geographic Origin-Specific Genotypic Differences and Phenotypic Plasticity. Wetlands 42, 113 (2022). https://doi.org/10.1007/s13157-022-01629-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-022-01629-4