Abstract
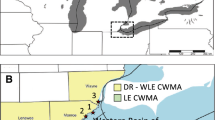
Constructed wetlands are being utilized to mitigate the impact that excess phosphorus in surface water has on the natural state of the Florida Everglades. This study investigates the role of aquatic metabolism in the retention of phosphorus in wetlands and how it varies with plant community. Eighteen 6-m2 mesocosms receiving inflows with relatively low phosphorus concentrations were planted with one of five wetland plant communities or left to natural colonization. In 2012, the mesocosms left to naturally colonize had significantly higher aquatic gross primary production (GPP) at 7.0 g O2 m−2 d−1 than all other communities. Mesocosms planted with Nymphaea odorata and those planted with a mix of Najas guadalupensis and Chara sp. had significantly higher GPP (5.5 and 5.9 g O2 m−2 d−1, respectively) than those with Typha domingensis, Eleocharis cellulosa, and Cladium jamaicense (1.7, 2.3, and 1.5 g O2 m−2 d−1, respectively). Rates of phosphorus cycling due to aquatic metabolism were estimated to range from 2.5 g P m−2 yr−1 in both the Cladium and Eleocharis communities to 7.7 g P m−2 yr−1in the naturally colonized mesocosms. These results provide evidence that wetland plant communities without high-biomass emergent macrophytes may perform best in the retention of phosphorus in low inflow concentration conditions.





Similar content being viewed by others
References
Ahn C, Mitsch WJ (2002) Scaling considerations of mesocosm wetlands in simulating large created freshwater marshes. Ecological Engineering 18: 327-342. 10.1016/S0925-8574(01)00092-1
American Public Health Association (APHA) (1998) Standard methods for the examination of water and wastewater, 20th edn. APHA, Washington D.C
Andersen JM (1975) An ignition method for determination of total phosphorus in lake sediments. Water Res 10:329–331. doi:10.1016/0043-1354(76)90175-5
Beadle C, Long S (1985) Photosynthesis - Is it limiting to biomass production. Biomass 8:119–168. doi:10.1016/0144-4565(85)90022-8
Brenner M, Hodell DA, Leyden BW, Curtis JH, Kenney WF, Gu BH, Newman JM (2006) Mechanisms for organic matter and phosphorus burial in sediments of a shallow, subtropical, macrophyte-dominated lake. J Paleolimnol 35:129–148. doi:10.1007/s10933-005-7881-0
Chimney M, Wenkert L, Pietro K (2006) Patterns of vertical stratification in a subtropical constructed wetland in south Florida (USA). Ecol Eng 27:322–330. doi:10.1016/j.ecoleng.2006.05.017
Cohen MJ, Kurz MJ, Heffernan JB, Martin JB, Douglass RL, Foster CR, Thomas RG (2013) Diel phosphorus variation and the stoichiometry of ecosystem metabolism in a large spring-fed river. Ecol Monogr 83:155–176. doi:10.1890/12-1497.1
Cole J, Pace ML, Carpenter SR, Kitchell JF (2000) Persistence of net heterotrophy in lakes during nutrient addition and food web manipulations. Limnol Oceanogr 45:1718–1730. doi:10.4319/lo.2000.45.8.1718
Coloso JJ, Cole JJ, Hanson PC, Pace ML (2008) Depth-integrated, continuous estimates of metabolism in a clear-water lake. Can J Fish Aquat Sci 65:712–722. doi:10.1139/F08-006
Cronk JK, Mitsch WJ (1994) Aquatic metabolism in 4 newly constructed fresh-water wetlands with different hydrologic inputs. Ecol Eng 3:449–468. doi:10.1016/0925-8574(94)00012-3
Fisher J, Acreman MC (1999) Wetland nutrient removal: a review of the evidence. Hydrol Earth Syst Sci 8:673–685. doi:10.5194/hess-8-673-2004
Fisher MM, Reddy KR (2001) Phosphorus flux from wetland soils affected by long-term nutrient loading. J Environ Qual 30:261–271. doi:10.2134/jeq2001.301261x
Gelda RK, Effler SW (2002) Estimating oxygen exchange across the air-water interface of a hypereutrophic lake. Hydrobiologia 487:243–254. doi:10.1023/A:1022994217578
Grimshaw HJ, Wetzel RG, Brandenburg M et al (1997) Shading of periphyton communities by wetland emergent macrophytes: Decoupling of algal photosynthesis from microbial nutrient retention. Arch Hydrobiol 139:17–27
Hagerthey SE, Cole JJ, Kilbane D (2010) Aquatic metabolism in the everglades: dominance of water column heterotrophy. Limnol Oceanogr 55:653–666. doi:10.4319/lo.2009.55.2.0653
Hall CAS, Moll R (1975) Methods of assessing aquatic primary productivity. In: Lieth H, Whittaker RH (eds) Primary productivity of the biosphere. Springer, New York, pp 19–53
Hansson L (1992) The role of food-chain composition and nutrient availability in shaping algal biomass development. Ecology 73:241–247. doi:10.2307/1938735
Hartley AM, House WA, Callow ME, Leadbeater BSC (1997) Coprecipitation of phosphate with calcite in the presence of photosynthesizing green algae. Water Res 31:2261–2268. doi:10.1016/S0043-1354(97)00103-6
House WA (1990) The prediction of phosphate coprecipitation with calcite in fresh-waters. Water Res 24:1017–1023. doi:10.1016/0043-1354(90)90124-O
International Organization for Standardization (ISO) (1995) ISO 10694:1995 Soil quality – Determination of organic and total carbon after dry combustion. ISO, Geneva
International Organization for Standardization (ISO) (1998) ISO 13878:1998 Soil quality –Determination of total nitrogen content by dry combustion. ISO, Geneva
Jansson M, Bergstrom A-K, Lymer D, Vrede K, Karlsson J (2006) Bacterioplankton growth and nutrient use efficiencies under variable organic carbon and inorganic phosphorus ratios. Microb Ecol 52:358–364. doi:10.1007/s00248-006-9013-4
Kadlec RH, Knight RL (1996) Treatment wetlands. Lewis Publishers, Boca Raton
Kufel L, Kufel I (2002) Chara beds acting as nutrient sinks in shallow lakes - a review. Aquat Bot 72:249–260. doi:10.1016/S0304-3770(01)00204-2
Maynard JJ, Dahlgren RA, O’Geen AT (2012) Quantifying spatial variability and biogeochemical controls of ecosystem metabolism in a eutrophic flow-through wetland. Ecol Eng 47:221–236. doi:10.1016/j.ecoleng.2012.06.032
Mitsch WJ, Gosselink JG (1986) Wetlands. VanNostrand Reinhold, New York
Mitsch WJ, Gosselink JG (2007) Wetlands, 5th edn. Wiley, Hoboken
Mitsch WJ, Kaltenborn KS (1980) Effects of copper sulfate application on diel dissolved oxygen and metabolism in the Fox Chain of Lakes. Trans Illi State Acad Sci 73:5δ5–64
Mitsch WJ, Cronk JK, Wu X, Nairn RW, Hey DL (1995) Phosphorus retention in constructed freshwater riparian marshes. Ecol Appl 5:830–845. doi:10.2307/1941991
Mitsch WJ, Horne AJ, Nairn RW (2000) Nitrogen and phosphorus retention in wetlands: ecological approaches to solving excess nutrient problems. Ecol Eng 14:1–7. doi:10.1016/S0925-8574(99)00015-4
Mitsch WJ, Gosselink JG, Anderson CJ, Zhang L (2009) Wetland ecosystems. Wiley, Hoboken, 295 pp
Mitsch WJ, Zhang L, Chung N, Marois D, Song, K, Villa JA (2013) Assessing nutrient removal efficacy and uptake of several native wetland plant communities. Final Report to South Florida Water Management District, Everglades Wetland Research Park, Florida Gulf Coast University, Naples, Florida, 48 pp. + appendices.
Mitsch WJ, Zhang L, Waletzko E, Bernal B (2014) Validation of the ecosystem services of created wetlands: two decades of plant succession, nutrient retention, and carbon sequestration in experimental riverine marshes. Ecological Engineering 72: 11-24. doi.org/10.1016/j.ecoleng.2014.09.108
Mitsch WJ, Zhang L, Marois DE, Song K (2015) Protecting the Florida Everglades wetlands with wetlands: can stormwater phosphorus be reduced to oligotrophic conditions? Ecological Engineering in press doi: 10.1016/j.ecoleng.2014.10.006
Newman S, Grace JB, Koebel JW (1996) Effects of nutrients and hydroperiod on typha, cladium, and eleocharis: implications for everglades restoration. Ecol Appl 6:774–783. doi:10.2307/2269482
Noe GB, Childers DL, Jones RD (2001) Phosphorus biogeochemistry and the impact of phosphorus enrichment: why is the everglades so unique? Ecosystems 4:603–624. doi:10.1007/s10021-001-0032-1
Odum EP (1969) Strategy of ecosystem development. Science 164:262–270. doi:10.1126/science.164.3877.262
Odum HT (1956) Primary production in flowing waters. Limnol Oceanogr 1(2):102–117. doi:10.4319/lo.1956.1.2.0102
Odum HT, Hoskin CM (1958) Comparative studies on the metabolism of marine waters. Publ Ma Sci Univ Texas 5:16–46
Pant HK, Reddy KR (2003) Potential internal loading of phosphorus in a wetland constructed in agricultural land. Water Res 37:965–972. doi:10.1016/S0043-1354(02)00474-8
Plummer LN, Wigley TML, Parkhurst DL (1978) The kinetics of calcite dissolution in CO2-water systems at 5° to 60 ° C and 0.0 to 1.0 atm CO2. Am J Sci 278:179–216. doi:10.2475/ajs.278.2.179
Reddy KR, DeLaune RD, DeBusk WF, Koch MS (1993) Long-term nutrient accumulation in the Everglades. (northern Everglades of Florida). Soil Sci Soc Am J 57:1147–1155. doi:10.2136/sssaj1993.03615995005700040044x
Reddy KR, Connor GAO, Gale PM (1998) Phosphorus sorption capacities of wetland soils and stream sediments impacted by dairy effluent. J Environ Qual 27:438–4473. doi:10.2134/jeq1998.00472425002700020027x
Reddy KR, Kadlec RH, Flaig E, Gale PM (1999) Phosphorus retention in streams and wetlands: a review. Crit Rev Environ Sci Technol 29:83–146. doi:10.1080/10643389991259182
Reeder BC (1994) Estimating the role of autotrophs in nonpoint-source phosphorus retention in a Laurentian Great-Lakes coastal wetland. Ecol Eng 3:161–169. doi:10.1016/0925-8574(94)90043-4
Reeder BC (2011) Assessing constructed wetland functional success using diel changes in dissolved oxygen, pH, and temperature in submerged, emergent, and open-water habitats in the Beaver Creek Wetlands Complex, Kentucky (USA). Ecol Eng 37:1772–1778. doi:10.1016/j.ecoleng.2011.06.018
South Florida Water Management District (SFWMD) (2013) Annual permit report for the Everglades stormwater treatment areas. 2013 South Florida environmental report. South Florida Water Management District, West Palm Beach, FL.
Staehr PA, Bade D, Van de Bogert MC et al (2010) Lake metabolism and the diel oxygen technique: state of the science. Limnol Oceanogr Methods 8:628–644. doi:10.4319/lom.2010.8.628
Talling JF (2010) pH, the CO2 system and freshwater science. Freshw Rev 3:133–146. doi:10.1608/FRJ-3.2.156
Tobias CR, Böhlke JK, Harvey JW (2007) The oxygen-18 isotope approach for measuring aquatic metabolism in high productivity waters. Limnol Oceanogr 52:1439–1453. doi:10.4319/lo.2007.52.4.1439
Tuttle CL, Zhang L, Mitsch WJ (2008) Aquatic metabolism as an indicator of the ecological effects of hydrologic pulsing in flow-through wetlands. Ecol Indic 8:795–806. doi:10.1016/j.ecolind.2007.09.005
Urban NH, Davis SM, Aumen NG (1993) Fluctuations in sawgrass and cattail densities in Everglades Water Conservation Area 2A under varying nutrient, hydrologic and fire regimes. Aquat Bot 46:203–223. doi:10.1016/0304-3770(93)90002-E
Van de Bogert MC, Carpenter SR, Cole JJ, Pace ML (2007) Assessing pelagic and benthic metabolism using free water measurements. Limnol Oceanogr Methods 5:145–155. doi:10.4319/lom.2007.5.145
Venkiteswaran JJ, Schiff SL, Wassenaar LI (2008) Aquatic metabolism and ecosystem health assessment using dissolved O-2 stable isotope diel curves. Ecol Appl 18:965–982. doi:10.1890/07-0491.1
Villa JA, Mitsch WJ, Song K, Miao SL (2014) Contribution of different wetland plant species to the DOC exported from a mesocosm experiment in the Florida Everglades. Ecol Eng 71:118–125. doi:10.1016/j.ecoleng.2014.07.011
Wahl M (2008) Ecological modulation of environmental stress: interactions between ultraviolet radiation, epibiotic snail embryos, plants and herbivores. J Anim Ecol 77:549–557. doi:10.1111/j.1365-2656.2007.01352.x
Acknowledgments
This study was supported by the South Florida Water Management District contract 4600001988 to The Ohio State University and continued by PO 4500070343 to Florida Gulf Coast University. The authors thank Evan Waletzko and DB Environmental for help with sampling and sample processing, Bob Johnson for designing the mesocosms’ hydraulic system, and the staff at the SFWMD laboratories for their help with lab processing and analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marois, D.E., Mitsch, W.J., Song, K. et al. Estimating the Importance of Aquatic Primary Productivity for Phosphorus Retention in Florida Everglades Mesocosms. Wetlands 35, 357–368 (2015). https://doi.org/10.1007/s13157-015-0625-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-015-0625-7