Abstract
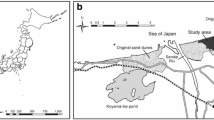
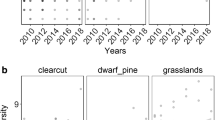
The preservation of endangered species requires clarifying habitat preferences through survival, growth and competitive ability. The determinants of habitat differentiation between the endangered species, Drosera anglica, and a widespread congener, D. rotundifolia, were compared. The effects of water level, Sphagnum mats and overstory vascular plants on Drosera distribution, recruitment and survival were monitored at a previously mined Sphagnum peatland. Seedling transplant experiments were conducted using different water levels. Seed-sowing experiments were conducted using different light intensities in three habitat-types: bare ground, Sphagnum mat and waterlogged surface. Distributions of D. anglica and D. rotundifolia were determined using survival at the seedling stage. D. anglica seedling recruitment and survival occurred more at lower water levels and/or lower plant cover, while D. rotundifolia seedlings established independent of these factors. In the greenhouse the seedlings of both species survived better at lower water levels but grew more slowly. D. anglica seedlings reduced their growth under shade more than D. rotundifolia. D. anglica showed low competitive light and nutrient ability on Sphagnum mats. Therefore, D. anglica was pushed to areas of high water levels where few competitors could establish. The habitat differentiation between D. anglica and D. rotundifolia originated from the interactions with Sphagnum mats.






Similar content being viewed by others
References
Adlassnig W, Peroutka M, Lambers H, Lichtscheidl IK (2005) The roots of carnivorous plants. Plant and Soil 274:127–140
Anderson JT, Landi AA, Marks PL (2009) Limited flooding tolerance of juveniles restricts the distribution of adults in an understory shrub (Itea virginica; Iteaceae). American Journal of Botany 96:1603–1611
Baskin CC, Milberg P, Andersson L, Baskin JM (2001) Seed dormancy-breaking and germination requirements of Drosera anglica, an insectivorous species of the Northern Hemisphere. Acta Oecologica-International Journal of Ecology 22:1–8
Braun-Blanquet J (1964) Pflanzensoziologie. Springer Verlag, Wien, New York
Brook BW, Sodhi NS, Bradshaw CJA (2008) Synergies among extinction drivers under global change. Trends in Ecology & Evolution 23:453–460
Charman D (2002) Peatlands and Environmental Change. John Wiley, Hoboken
Coops H, van der Velde G (1995) Seed dispersal, germination and seedling growth of six hetelophyte species in relation to water-level zonation. Freshwater Biology 34:13–20
Crowder AA, Pearson MC, Grubb PJ, Langlois PH (1990) Biological flora of the British Isles. Drosera L Journal of Ecology 78:233–267
Dalling JW, Winter K, Nason JD, Hubbell SP, Murawski DA, Hamrick JL (2001) The unusual life history of Alseis blackiana: a shade-persistent pioneer tree? Ecology 82:933–945
Egawa C, Koyama A, Tsuyuzaki S (2009) Relationships between the developments of seedbank, standing vegetation and litter in a post-mined peatland. Plant Ecology 203:217–228
Eriksson O (2002) Ontogenetic niche shifts and their implications for recruitment in three clonal Vaccinium shrubs: Vaccinium myrtillus, Vaccinium vitis-idaea, and Vaccinium oxycoccos. Canadian Journal of Botany 80:635–641
Fenton NJ, Bergeron Y (2006) Facilitative succession in a boreal bryophyte community driven by changes in available moisture and light. Journal of Vegetation Science 17:65–76
Gelman A, Carlin JB, Stern HS, Rubin DB (2003) Bayesian data analysis. Chapman and Hall, London, UK
Gore AJP (ed) (1983) Mires: swamp, bog, fen and moor. Ecosystems of the World 4A. Elsevier, Amsterdam
Griffith AB, Forseth IN (2003) Establishment and reproduction of Aeschynomene virginica (L.) (Fabaceae) a rare, annual, wetland species in relation to vegetation removal and water level. Plant Ecology 167:117–125
Heijmans MMPD, Klees H, Berendse F (2002) Competition between Sphagnum magellanicum and Eriophorum angustifolium as affected by raised CO2 and increased N deposition. Oikos 97:415–425
Hoyo Y, Tsuyuzaki S (2013) Characteristics of leaf shapes among two parental Drosera species and a hybrid examined by canonical discriminant analysis and a hierarchical Bayesian model. American Journal of Botany 100:817–823
Huntke T (2007) The distribution of Drosera anglica Huds. in lower Saxony past and present–the extent of the decline of a raised bog specialist and its causes. Tuexenia: 241–253
IUCN Red List of Threatened Species. Version 2013. http://www.iucnredlist.org/
Japan Meteorological Agency (2012). http://www.jma.go.jp/jma/(in Japanese)
Jennings DE, Rohr JR (2011) A review of the conservation threats to carnivorous plants. Biological Conservation 144:1356–1363
Keddy PA, Fraser LH, Wisheu IC (1998) A comparative approach to examine competitive responses of 48 wetland plant species. Journal of Vegetation Science 9:777–786
Keddy PA (2010) Wetland ecology: Principles and Conservation. Cambridge University Press, Cambridge, UK
Koyama A, Tsuyuzaki S (2010) Effects of sedge and cottongrass tussocks on plant establishment patterns in a post-mined peatland, northern Japan. Wetlands Ecology and Management 18:135–148
Koyama A, Tsuyuzaki S (2013) Facilitation by tussock-forming species on seedling establishment collapses in an extreme drought year in a post-mined Sphagnum peatland. Journal of Vegetation Science 24:473–483
Malmer N, Svensson BM, Wallén B (1994) Interactions between Sphagnum mosses and field layer vascular plants in the development of peat-forming systems. Folia Geobotanica et Phytotaxonomica 29:483–496
Malmer N, Albinsson C, Svensson BM, Wallén B (2003) Interferences between Sphagnum and vascular plants: effects on plant community structure and peat formation. Oikos 100:469–482
Ministry of the Environment Government of Japan (2013) Japan integrated biodiversity information system. Red list of Threatened Plant of Japan, revisedth edn. Ministry of the Environment Government of Japan, Tokyo, Japan
Murray BR, Thrall PH, Gill AM, Nicotra AB (2002) How plant life-history and ecological traits relate to species rarity and commonness at varying spatial scales. Austral Ecology 27:291–310
Nishimura A, Tsuyuzaki S, Haraguchi A (2009) A chronosequence approach for detecting revegetation patterns after Sphagnum-peat mining, northern Japan. Ecological Research 24:237–246
Nordbakken JF (1996) Plant niches along the water-table gradient on an ombrotrophic mire expanse. Ecography 19:114–121
Nordbakken JF, Rydgren K, Økland RH (2004) Demography and population dynamics of Drosera anglica and D. rotundifolia. Journal of Ecology 92:110–121
Poorter L (2007) Are species adapted to their regeneration niche, adult niche, or both? The American Naturalist 169:433–442
Rivadavia F, Kondo K, Kato M, Hasebe M (2003) Phylogeny of the sundews, Drosera (Droseraceae), based on chloroplast rbcL and nuclear 18S ribosomal DNA sequences. American Journal of Botany 90:123–130
Rochefort L (2000) New frontiers in bryology and lichenology–Sphagnum–A keystone genus in habitat restoration. Bryologist 103:503–508
Römermann C, Tackenberg O, Jackel AK, Poschiod P (2008) Eutrophication and fragmentation are related to species’ rate of decline but not to species rarity: results from a functional approach. Biodiversity and Conservation 17:591–604
Sosnová M, van Diggelen R, Klimešová J (2010) Distribution of clonal growth forms in wetlands. Aquatic Botany 92:33–39
Spiegelhalter D, Thomas A, Best N, Lunn D (2003) WinBUGS version 1.4 User manual. http://www.mrc-bsu.cam.ac.uk/bugs
Svensson BM (1995) Competition between Sphagnum fuscum and Drosera rotundifolia: a case of ecosystem engineering. Oikos 74:205–212
ten Brink D-J, Hendriksma HP, Bruun HH (2013) Habitat specialization through germination cueing: a comparative study of herbs from forests and open habitats. Annals of Botany 111:283–292
Tsuyuzaki S, Sawada Y, Kushida K, Fukuda M (2008) A preliminary report on the vegetation zonation of palsas in the Arctic National wildlife Refuge, northern Alaska, USA. Ecological Research 23:787–793
van Breemen N (1995) How Sphagnum bogs down other plants. Trends in Ecology and Evolution 10:270–275
Verhulst J, Montana C, Mandujano MC, Franco M (2008) Demographic mechanisms in the coexsistence of two closely related perennials in a fluctuating environment. Oecologia 156:95–105
Wolf E, Gage E, Cooper DJ (2006) Drosera anglica Huds. (English sundew): A technical conservation assessment. Report prepared for the USDA Forest Service, Rocky Mountain Region, Species Conservation Project [online]. Website http://www.fs.usda.gov/ Internet/FSE_DOCUMENTS/stelprdb5250872.pdf./[accessed 30 August 2012]
Young AS, Chang SM, Sharitz RR (2007) Reproductive ecology of a federally endangered legume, Baptisia arachnifer and its more widespread congener, B. lanceolata (Fabaceae). American Journal of Botany 94:228–236
Zietsman J, Dreyer LL, Esler KJ (2008) Reproductive biology and ecology of selected rare and endangered Oxalis L. (Oxalidaceae) plant species. Biological Conservation 141:1475–1483
Acknowledgments
We wish to thank members of the plant ecology laboratory for their support, and T. Kubo for his statistical analyses. We are grateful to staff from the Ministry of Environment of Japan and the Toyotomi Town Office for research permission and support. This work is partly supported by grants from Japan Society for the Promotion of Science, Expo '90 Foundation and Global COE grants to Hokkaido University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoyo, Y., Tsuyuzaki, S. Habitat Differentiation Between Drosera anglica and D. rotundifolia in a Post-Mined Peatland, Northern Japan. Wetlands 34, 943–953 (2014). https://doi.org/10.1007/s13157-014-0555-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-014-0555-9