Abstract
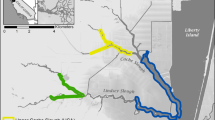
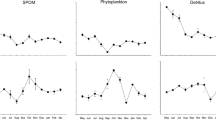
Salt marsh restoration is hypothesized to provide shoreline stabilization, increased fish habitat, and organic carbon subsidies for estuarine food webs. Organic carbon comes from diverse primary producers that differ in carbon fixation rates and areal extent within wetland systems. This study was designed to obtain some of the first estimates of the relative contribution of different primary producers to total organic carbon production within open water and tidally flooded wetlands of the northern San Francisco Estuary (SFE). Carbon fixation rates of phytoplankton, microphytobenthos, and low marsh emergent vegetation were measured in two natural and four restoring wetlands in 2004. Areal (m2) rates of carbon fixation were greatest for low marsh vegetation, while phytoplankton and microphytobenthos rates were one and two orders of magnitude lower, respectively. However, when areal production rates were scaled to the amount of habitat available for each primary producer group, the relative importance of each group varied by location. Given that each primary producer group supports a different subset of estuarine consumers, the type of food subsidy desired should influence the amount open water channel, mudflat and low marsh area restored. Large-scale wetland restoration activities should consider the types of primary producers likely to occupy restored habitats when estimating future food web impacts.





Similar content being viewed by others
References
Admiraal W, Peletier H, Zomer H (1982) Observations and Experiments on the Population Dynamics of Epipelic Diatoms from an Estuarine Mudflat. Estuar Coast Shelf Sci 14:471–487
Alpine AE, Cloern JE (1992) Trophic Interactions and Direct Physical Effects Control Phytoplankton Biomass and Production in an Estuary. Limnol Oceanogr 37:946–955
Boesch DF, Turner RE (1984) Dependence of Fishery Species on Salt Marshes: the Role of Food and Refuge. Estuar Coast Shelf Sci 7:460–468
Boyer T, Polasky S (2004) Valuing Urban Wetlands: a Review of non-Market Valuation. Wetl 24:744–755
Bran and Luebbe AutoAnalyzer Applications (1999) AutoAnalyzer Method No. G–177–96 Silicate in water and seawater Bran Luebbe, Inc. Buffalo Grove, IL
Bronk DA, See JH, Bradley P, Killberg L (2006) DON as a Source of Bioavailable Nitrogen for Phytoplankton. Biogeosci Discuss 3:1247–1277
Brown LR (2003a) An Introduction to the San Francisco Estuary Tidal Wetlands Restoration Series. San Francisco Estuary Watershed Sci 1:1–10
Brown LR (2003b) Will Tidal Wetland Restoration Enhance Populations of Native Fishes? San Francisco Estuary and Watershed Sci 1:10–54
Bucholz JW (1982) Nitrogen flux between a developing salt marsh and South San Francisco Bay. MA thesis, San Francisco State University, San Francisco, CA
Burdick DM, Mendelssohn IA, McKee KL (1989) Live Standing Crop Metabolism of the Marsh Grass Spartina patens as Related to Edaphic Factors in a Brackish Mixed Marsh Community in Louisiana. Estuar Coast Shelf Sci 12:195–204
Buzzelli CP, Wetzel RL (1998) Dynamic Simulation of Littoral Zone Habitats in Lower Chesapeake Bay. II Seagrass Habitat Primary Production and Water Quality Relationships. Estuaries 21:673–689
Callaway JC, Parker VT, Vasey MC, Schile LM (2007) Emerging Issues for the Restoration of Tidal Marsh Ecosystems in the Context of Predicted Climate Change. Madrono 54:234–248
Callaway JC, Bornis EL, Turner RE, Milan CS (2012) Carbon Sequestration and Sediment Accretion in San Francisco Bay Tidal Wetlands. Estuar Coasts 35:1163–1181
Chambers RM, Harvey JW, Odum WE (1992) Ammonium and Phosphate Dynamics in a Virginia Salt Marsh. Estuar Coast Shelf Sci 15:349–359
Chmura GL, Anisfeld SC, Cahoon DR, Lynch JC (2003) Global Carbon Sequestration in Tidal, Saline Wetland Soils. Glob Biogeochem Cycles 17:1111–1124
Cloern JE (1987) Turbidity as a Control on Phytoplankton Biomass and Productivity in Estuaries. Cont Shelf Res 7:1367–1381
Cloern JE, Canuel EA, Harris D (2002) Stable Carbon and Nitrogen Isotope Composition of Aquatic and Terrestrial Plants of the San Francisco Bay Estuarine System. Limnol Oceanogr 47:713–729
Cole BE, Cloern JE (1984) Significance of Biomass and Light Availability to Phytoplankton Productivity in San Francisco Bay. Mar Ecol Prog Ser 17:15–24
Colijn F, de Jonge VN (1984) Primary Production of Microphytobenthos in the Ems-Dollard Estuary. Mar Ecol Prog Ser 14:185–196
Cramer GW, Day JW, Conner WH (1981) Productivity of Four Marsh Sites Surrounding Lake Pontchartrain, Louisiana. Am Midl Nat 106:65–72
Darby FA, Turner RE (2008) Below and Aboveground Spartina alterniflora Production in a Louisiana Salt Marsh. Estuar Coasts 31:223–231
Day JW, Britsch LD, Hawes SR, Shafer GP, Reed DJ, Cahoon D (2000) Pattern and Process of Land Loss in the Mississippi Delta: a Spatial and Temporal Analysis of Wetland Habitat Change. Estuar Coast Shelf Sci 23:425–438
de Jonge VN, Colijn F (1994) Dynamics of microphytobenthos biomass in the Ems Estuary. Mar Ecol Prog Ser 104:85–196
Erwin KL (2009) Wetlands and Global Climate Change: the Role of Wetland Restoration in a Changing World. Wetl Ecol Manag 17:71–84
Espanol C, Gallardo B, Pino MR, Martin A, Comin FA (2013) Is net Ecosystem Production Higher in Natural Relative to Constructed Wetlands? Aquat Sci 75:385–397
Finlayson CM, Davidson NC, Spiersand AG, Stevenson NJ (1999) Global Wetland Inventory Current Status and Future Priorities. Mar Freshw Res 50:717–727
Friederich GE, Walz PM, Burczynski MG, Chavez FP (2002) Inorganic Carbon in the Central California Upwelling System During the 1997–1999 El Niño – La Niña Event. Prog Oceanogr 54:185–203
Gallagher JL, Daiber FC (1974) Primary Production of Edaphic Algal Communities in a Delaware Salt Marsh. Limnol Oceanogr 19:390–395
Galvan K, Fleeger JW, Peterson B, Drake D, Deegan LA, Johnson DS (2011) Natural Abundance Stable Isotopes and Dual Isotope Tracer Additions Help to Resolve Resources Supporting a Saltmarsh Food web. J Exp Mar Biol Ecol 410:1–11
Geider RJ, Osborne BA (1992) Algal photosynthesis. Chapman & Hall, New York
Glibert PM, Fullerton D, Burkholder JM, Cornwell JC, Kana TM (2011) Ecological Stoichiometry, Biogeochemical Cycling, Invasive Species, and Aquatic Food: Webs San Francisco Estuary and Comparative Systems. Rev Fish Sci 19:358–417
Gould DM, Gallagher ED (1990) Field Measurement of Specific Growth Rate, Biomass, and Primary Production of Benthic Diatoms of Savin Hill Cove, Boston. Limnol Oceanogr 5:1757–1770
Grimaldo L, Hymanson Z (1999) What is the Impact of the Introduced Brazilian Waterweed Egeria densa to the Delta Ecosystem? Interagency Ecol Program Newsl 12:43–45
Grimaldo LF, Stewart AR, Kimmerer W (2009) Dietary Segregation of Pelagic and Littoral Fish Assemblages in a Highly Modified Tidal Freshwater Estuary. Mar and Coast Fish:Dyn, Manag, and Ecosyst Sci 1:200–217
Harding LW, Mallonee ME, Perry ES (2002) Toward a Predictive Understanding of Primary Productivity in a Temperate, Partially Stratified Estuary. Estuar Coast Shelf Sci 55:437–463
Hickson D, Keeler-Wolf T (2007) Vegetation and land use classification and map of the Sacramento-San Joaquin River Delta. California department of fish and game. 283 pp
Howe ER, Simenstad CA (2007) Restoration Trajectories and Food web Linkages in San Francisco Bay’s Estuarine Marshes: a Manipulative Translocation Experiment. Mar Ecol Prog Ser 351:65–76
Howe ER, Simenstad CA (2011) Isotopic Determination of Food web Origins in Restoring and Ancient Wetlands of the San Francisco Bay and Delta. Estuar Coasts 34:597–617
IOC (Intergovernmental Oceanographic Commission) (1996) JGOFS Report 19. Protocols for the joint global ocean flux study (JGOFS) core measurements
Jassby AD, Cloern JE (2000) Organic Matter Sources and Rehabilitation of the Sacramento-San Joaquin Delta (California, USA). Aquat Conserv Mar Freshwat Ecosyst 10:323–352
Jassby AD, Cloern JE, Powell TM (1993) Organic Carbon Sources and Sinks in San Francisco Bay: Variability Induced by River Flow. Mar Ecol Prog Ser 95:39–54
Josselyn MN, West JA (1985) The Distribution and Temporal Dynamics of the Estuarine Macroalgal Community of San Francisco Bay. Hydrobiologia 129:139–152
Kennish MJ (2001) Coastal Salt Marsh Systems in the US: a Review of Anthropogenic Impacts. J Coast Res 17:731–748
Kimmerer WJ, Parker AE, Lidstrom U, Carpenter EJ (2012) Short-Term and Interannual Variability in Primary Productivity in the low-Salinity Zone of the San Francisco Estuary. Estuar Coasts. doi:10.1007/s12237–012–9482-2
Leach JH (1970) Epibenthic Algal Production in an Intertidal Mudflat. Limnol Oceanogr 15:514–521
Littler MM, Littler DS (1985) Ecological Field Methods: Macroalgae. In: Littler MM, Littler DS (eds) Handbook of Phycological Methods. Cambridge Univ. Press, New York
Lorenzi A (2006) Primary Productivity and rbcL gene expression in Central San Francisco Bay. MS thesis, San Francisco State University, San Francisco, CA
Madsen JD (1993) Biomass Techniques for Monitoring and Assessing Control of Aquatic Vegetation. Lake and Reserv Manag 7:141–154
Nobriga ML, Feyrer F, Baxter RD, Chotkowski M (2005) Fish Community Ecology in an Altered River Delta: Spatial Patterns in Species Composition, Life-History Strategies, and Biomass. Estuar Coast Shelf Sci 28:776–785
Orr M, Crooks S, Williams PB (2003) Will restored tidal marshes be sustainable? In: Brown LR (ed) Issues in San Francisco Estuary tidal wetlands restoration. San Francisco Estuary and Watershed Science 1:Article 5
Parker AE, Fuller J, Dugdale RC (2006) Estimating dissolved inorganic carbon concentrations from salinity in San Francisco Bay for use in 14C- primary production studies. Interagency Ecol Prog Newsl 19:17–22
Parker AE, Hogue VE, Wilkerson FP, Dugdale RC (2012) The Effect of Inorganic Speciation on Primary Production in the San Francisco Estuary. Estuar Coast Shelf Sci 104:91–101
Pearcy RW, Ustin SL (1984) Effects of Salinity on Growth and Photosynthesis of Three California Tidal Marsh Species. Oecologia 62:68–73
Peckham SD, Chipman JW, Lillesand TM, Dodson SI (2006) Alternate Stable States and the Shape of Lake Trophic Distribution. Hydrobiologia 571:401–407
Peterson BJ, Howarth RW (1987) Sulfur, Carbon and Nitrogen Isotopes Used to Trace Organic Matter Flow in the Salt-Marsh Estuaries of Sapelo Island, Georgia. Limnol Oceanogr 32:1195–1213
Pinckney J, Zingmark RG (1993) Modeling the Annual Production of Intertidal Benthic Microalgae in Estuarine Ecosystems. J Phycol 29:396–407
Pinckney JL, Carman KR, Lumsden SE, Hymel SN (2003) Microalgal-Meiofaunal Trophic Relationships in Muddy Intertidal Estuarine Sediments. Aquat Microb Ecol 31:99–108
Riera P, Stal LJ, Nieuwenhuize J, Richard P, Blanchard G, Gentil F (1999) Determination of Food Sources for Benthic Invertebrates in a Salt Marsh (Aiguillon Bay, France) by Carbon and Nitrogen Stable Isotopes: Importance of Locally Produced Sources. Mar Ecol Prog Ser 187:301–307
Roman CT, Able KW, Lazzari MA, Heck KL (1990) Primary Productivity of Angiosperm and Macroalgae Dominated Habitats in a New England Salt Marsh: a Comparative Analysis. Estuar Coast Shelf Sci 30:35–46
Scheffer M, Szabo S, Gragnani A, van Nes EH, Rinaldi S, Kautsky N, Norberg J, Roijackers RMM, Franken RJM (2003) Floating Plant Dominance as a Stable State. Proc of the Natl Acad of Sci of the U S A 100:4040–4045
Smart RM (1982) Distribution and Environmental Control of Productivity and Growth Form of Spartina alterniflora (Loisel.). Tasks for Vegetation Sci 2:127–142
Sobczak WV, Cloern JE, Jassby AD, Muller-Solger AB (2002) Bioavailability of Organic Matter in a Highly Disturbed Estuary: The Role of Detrital and Algal Sources. Proc Natl Acad Sci U S A 99:8101–8105
Solorzano L (1969) Determination of Ammonia in Natural Waters by the Phenolhypochlorite Method. Limnol Oceanogr 14:799–801
Sullivan MJ, Currin CA (2000) Community structure and functional dynamics of benthic microalgae in salt marshes. In: Weinstein MP, Kreeger DA (eds) Concepts and Controversies in Tidal Marsh Ecology. Kluwer Academic Publishers, Dordrecht, pp 81–106
Tu M, Randall JM (2001) 2001 red Alert! New Expansions into and Around California. Calif Exotic Pest Coun 9:4–5
Underwood AJ (1997) Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance. Cambridge University Press, Cambridge
Van Raalte C, Stewart WC, Valiella I, Carpenter EJ (1974) A 14C Technique for Measuring Algal Productivity in Salt Marsh Muds. Bot Mar 17:186–188
Van Raalte CD, Valiela I, Teal JM (1976) Production of Epibenthic Salt Marsh Algae: Light and Nutrient Limitation. Limnol Oceanogr 21:862–872
Varela M, Penas E (1985) Primary Production of Benthic Microalgae in an Intertidal Sand Flat of the Ria de Arosa, NW Spain. Mar Ecol Prog Ser 25:111–119
Wainright SC, Weinstein MP, Able KW, Currin CA (2000) Relative Importance of Benthic Microalgae, Phytoplankton and the Detritus of Smooth Cordgrass Spartina Alterniflora and the Common Reed Phragmites australis to Brackish-Marsh Food Webs. Mar Ecol Prog Ser 200:77–91
Warren RS, Fell PE, Roszsa R, Brawley AH, Orsted AC, Olsen ET, Swamy V, Niering WA (2002) Salt Marsh Restoration in Connecticut: 20 Years of Science and Management. Restor Ecol 10:497–513
Wetlands and Water Resources, Inc. (2012) Integrative Regional Wetland Monitoring (IRWM) Study Site Characterization Report, March, 2012, http://www.swampthing.org/, 207 pp
Whiting GJ, Chanton JP (2001) Greenhouse Carbon Balance of Wetlands: Methane Emission Versus Carbon Sequestration. Tellus 53B:521–528
Whitledge TE, Malloy SC, Patton CJ, Wirick CD (1981) Automated Nutrient Analysis in Seawater, Report BNL 51398. Brookhaven National Laboratory, Upton, p 216
Wilkerson FP, Dugdale RC, Hogue VE, Marchi A (2006) Phytoplankton Blooms and Nitrogen Productivity in San Francisco Bay. Estuar Coast Shelf Sci 29:401–416
Zedler JB (1996) Tidal wetland restoration: a scientific perspective and southern California focus. California Sea Grant College System, University of California, La Jolla
Zedler JB, Kercher S (2005) Wetland Resources: Status, Ecosystem Services, Degradation, and Restorability. Annu Rev Environ Resour 30:39–74
Acknowledgments
This material is based upon work supported by the CALFED Science Program under Grant No. 4600002883 and the Association of Bay Area Governments ERP grant E1083005/ABAG project #102186. Access to sites was granted by CA DFW and the East Bay Regional Park District. Many thanks to A. Marchi for performing water nutrient analyses, and to C. R. Chandler for providing advice on statistical analyses. Assistance with field sampling and laboratory analyses was provided by P. Bouley, S. Govil, J. Hausmann, C. Little, W. Most, A. Slaughter, K. Walker. Comments from P. Glibert, J. Cornwell and two anonymous reviewers greatly improved the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 47 kb)
Rights and permissions
About this article
Cite this article
Cohen, R.A., Wilkerson, F.P., Parker, A.E. et al. Ecosystem-Scale Rates of Primary Production Within Wetland Habitats of the Northern San Francisco Estuary. Wetlands 34, 759–774 (2014). https://doi.org/10.1007/s13157-014-0540-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-014-0540-3