Abstract
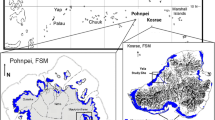
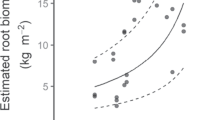
Root production influences a range of belowground processes, such as soil accretion, carbon sequestration and nutrient acquisition. Here, we measured biomass and root production of mangroves surrounding a karstic oligotrophic lagoon that spans a nutrient and salinity gradient. We also measured forest structure and soil physicochemical conditions (salinity, bulk density, carbon, nitrogen (N) and phosphorus (P)) in order to determine factors associated with root production. We tested the following hypotheses: 1) root biomass and production increase at low soil P and N in order to maximize resource utilization, and 2) root biomass and production increase with high interstitial salinity. Root biomass (947–3,040 g m−2) and production (0.46–1.85 g m−2 day−1) increased where soil P and interstitial salinity were relatively high. Thus, we rejected the first hypothesis and confirmed the second. The larger root fraction (5–20 mm) was the major contributor to root biomass and production. Our findings suggest that root production and thus capacity for belowground carbon storage in karstic regions, where P is often limiting, is greater where interstitial salinity and P are higher. This contrasts with past assessments indicating that P-deficiency stimulates root growth, suggesting wide variation in belowground responses in mangroves.




Similar content being viewed by others
References
Adame MF, Zaldívar-Jiménez A, Teutli C, Caamal JP, Andueza M, Lopez-Adame A, Cano R, Hernandez-Arana HA, Torres-Lara R, Herrera-Silveira JA (2012) Drivers of mangrove litterfall within a karstic region affected by frequent hurricanes. Biotropica 45:147–154
Adame MF, Kauffman JB, Medina I, Gamboa JN, Torres O, Caamal J, Herrera-Silveira J (2013) Carbon stocks of tropical coastal wetlands within the karstic landscape of the Mexican Caribbean. PLoS ONE 8:e56569
Alongi DM (2011) Carbon payment for mangrove conservation: constraints and uncertainties of sequestration potential. Environmental Science & Policy 14:462–470
ArandaCicerol N, Herrera-Silveira JA, Comín FA (2006) Nutrient water quality in a tropical coastal zone with groundwater discharge, northwest Yucatan, Mexico. Estuarine, Coastal and Shelf Science 68:445–454
Aspila KI, Agemian H, Chau SY (1976) A semi-automated method for determination of inorganic, organic and total phosphate in sediments. Analyst 101:187–197
Ball MC (1988) Salinity tolerance in the mangroves Aegiceras corniculatum and Avicennia marina. I. Water use in relation to growth, carbon partitioning and salt balance. Australian Journal of Plant Physiology 15:447–464
Ball MC (2002) Interactive effects of salinity and irradiance on growth: implications for mangrove forest structure along salinity gradients. Trees 16:126–139
Ball MC, Cochrane MJ, Rawson HM (1997) Growth and water use of the mangroves, Rhizophora apiculata and R. stylosa in response to salinity and humidity under ambient and elevated concentrations of atmospheric CO2. Plant, Cell and Environment 20:1158–1166
Cahoon DR, Hensel P, Rybczyk J, McKee KL, Proffitt CE, Perez BC (2003) Mass tree mortality leads to mangrove peat collapse at Bay Islands, Honduras after Hurricane Mitch. Journal of Ecology 91:1093–1105
Castañeda-Moya E, Twilley RR, Rivera-Monroy VH, Marx BD, Coronado-Molina C, Ewe SML (2011) Patterns of root dynamics in mangrove forests along environmental gradients in the Florida coastal Everglades, USA. Ecosystems 14:1178–1195
Chapin SF (1991) Integrated responses of plants to stress. BioScience 41:29–36
Cintrón G, Schaeffer Novelli Y (1984) Methods for studying mangrove structure. In: Snedaker SC, Snedaker JG (eds) The mangrove ecosystem: research methods. UNESCO, Paris, pp 91–113
Clark DA, Brown S, Kicklighter DW, Chambers JQ, Thomlinson JR, Ni J (2001) Measuring net primary production in forests: concepts and field methods. Ecological Applications 11:356–370
Clough B (1992) Mangrove productivity and growth of mangrove forests. In: Roberstson AI, Alongi DM (eds) Tropical mangrove ecosystems. American Geophysical Union, Washington, pp 225–249
CONABIO (2009) Manglares de México: Estensión y distribución, 2nd edn. National Comission on the Knowledge and Use of Biodiversity (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad) Federal Government, Mexico, p 99
Dahdouh-Guebas F, Koedam N (2006) Empirical estimate of the reliability of the use of the Point-Centred Quarter Method (PCQM): solutions to ambiguous field situations and description of the PCQM+ protocol. Forest Ecology and Management 228:1–18
Feller IC, Whigham DF, McKee KL, Lovelock CE (2003) Nitrogen limitation of growth and nutrient dynamics in a disturbed mangrove forest, Indian River Lagoon, Florida. Oecologia 134:405–414
García E, Mosiño P (1992) Los climas de México, vol. 2. Instituto de Geografía. UNAM, Mexico City
Gleason SM, Ewel KC (2002) Organic matter dynamics on the forest floor of a Micronesian mangrove forest: an investigation of species composition shifts. Biotropica 34:190–198
Grieve AP (1984) Tests of sphericity of normal distributions and the analysis of repeated measurements designs. Psychometrika 49:257–267
Herrera-Silveira JA (1996) Salinity and nutrients in a tropical coastal lagoon with groundwater discharges to the Gulf of Mexico. Hydrobiologia 321:165–176
Herrera-Silveira JA, Morales-Ojeda SM (2009) Evaluation of the health status of a coastal ecosystem in southeast Mexico: assessment of water quality, phytoplankton and submerged aquatic vegetation. Marine Pollution Bulletin 59:72–86
Herrera-Silveira JA, Teutli HC, Zaldívar JA, Pérez CR, Cortés BO, Ramírez RJ, Alvarado E, Caamal-Sosa J, Andueza T, Hernández AH (2010) Programa regional para la caracterización y el monitoreo de ecosistemas de manglar del Golfo de México y el Caribe Mexicano: Inicio de una red multi-institucional. Yucatan Peninsula. CINVESTAV-ECOPEY/CONABIO FB1307-N009/08 4th Report. Yucatan, México, p 48
Komiyama A, Ogino K, Aksornkoae S, Sabhasri S (1987) Root biomass of a mangrove forest in southern Thailand. 1 Estimation by the trench method and the zonal structure of root biomass. Journal of Tropical Ecology 3:97–108
Komiyama A, Havanond S, Srisawatt W, Mochida Y, Fujimoto K, Ohnishi T, Ishihara S, Miyagi T (2000) Top/root biomass ratio of a secondary mangrove (Ceriops tagal (Perr.) C.B. Rob.) forest. Forest Ecology and Management 139:127–134
Komiyama A, Poungparn S, Kato S (2005) Common allometric equations for estimating the tree weight of mangroves. Journal of Tropical Ecology 21:471–477
Krauss KW, McKee KL, Lovelock CE, Cahoon DR, Saintilan N, Reef R, Chen L (2013) How mangrove forests adjust to rising sea level. New Phytologist
López B, Sabaté S, Gracia C (1998) Fine root dynamics in a Mediterranean forest: effects of drought and stem density. Tree Physiology 18:601–606
Lovelock CE, Ball MC, Feller IC, Engelbrecht BMJ, Ewe ML (2006a) Variation in hydraulic conductivity of mangroves: influence of species, salinity, and nitrogen and phosphorus availability. Physiologia Plantarum 127:457–464
Lovelock CE, Ball MC, Choar B, Engelbrecht BMJ, Holbrook NM, Feller IC (2006b) Linking physiological processes with mangrove forest structure: phosphorus deficiency limits canopy development, hydraulic conductivity and photosynthetic carbon gain in dwarf Rhizophora mangle. Plant, Cell and Environment 29:793–802
Lovelock CE, Ruess RW, Feller IC (2006c) Fine root respiration in the mangrove Rhizophora mangle over variation in forest stature and nutrient availability. Tree Physiology 26:1601–1606
Lovelock CE, Ball MC, Martin KC, Feller IC (2009) Nutrient enrichment increases mortality of mangroves. PLoS ONE 4:e5600
Matsui N (1998) Estimated stocks of organic carbon in mangrove roots and sediments in Hinchbrook Channel, Australia. Mangroves and Salt Marshes 2:199–204
McKee KL (2001) Root proliferation in decaying roots and old root channels: a nutrient conservation mechanism in oligotrophic mangrove forests? Journal of Ecology 89:876–887
McKee KL, Faulkner PL (2000) Restoration of biogeochemical function in mangrove forests. Restoration Ecology 8:247–259
McKee KL, Cahoon DR, Feller IC (2007) Caribbean mangroves adjust to rising sea level through biotic controls on change in soil elevation. Global Ecology and Biogeography 6:545–556
Naidoo G (2009) Differential effects of nitrogen and phosphorus enrichment on growth of dwarf Avicennia marina mangroves. Aquatic Botany 90:184–190
Nye PH, Tinker PB (1977) Soluble movement in the soil root system. Blackwell Publishers, Oxford
NOAA (National Ocean and Atmospheric Administration) (2011) Historical hurricane tracks, Washington, D.C. Available at: http://csc.noaa.gov/hurricanes/. Accessed 23 March 2011
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, New York
Perry E, Paytan A, Pedersen B, Velazquez-Oliman G (2009) Groundwater geochemistry of the Yucatan Peninsula, Mexico: constraints on stratigraphy and hydrogeology. Journal of Hydrology 367:27–40
Poret N, Twilley RR, Rivera-Monroy VH, Coronado-Molina C (2007) Belowground decomposition of mangrove roots in Florida coastal everglades. Estuaries and Coasts 30:491–496
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Saintilan N (1997) Above-and below-ground biomasses of two species of mangrove on the Hawkesbury River estuary, New South Wales. Marine and Freshwater Research 48:147–152
Vogt KA, Vogt DJ, Bloomfield J (1998) Analysis of some direct and indirect methods for estimating root biomass and production of forests at an ecosystem level. Plant and Soil 200:71–89
Zaldívar-Jiménez A, Silveira-Herrera J, Coronado-Molina C, Alonzo-Parra D (2004) Estructura y productividad de los manglares en la reserva de la biosfera Ría Celestún, Yucatán, México. Maderas y Bosques 2:23–25
Acknowledgments
We want to thank The Mexican Council of Science and Technology (CONACyT), The Research Centre and Postgraduate Studies of the National Polytechnic Institute (CINVESTAV, Mérida) and the Australian Rivers Institute at Griffith University. We are grateful for field support to the National Commission for Natural Protected Areas (CONANP). We thank Ileana Osorio for laboratory assistance and Dr. Timothy Mercer and Dr. Daniel Klein for editing assistance. We also want to thank the anonymous reviewers for their insightful comments that helped us improve the manuscript. This work was partially supported by a grant from the National Commission on Biodiversity (CONABIO FN009).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(DOC 47 kb)
Rights and permissions
About this article
Cite this article
Adame, M.F., Teutli, C., Santini, N.S. et al. Root Biomass and Production of Mangroves Surrounding a Karstic Oligotrophic Coastal Lagoon. Wetlands 34, 479–488 (2014). https://doi.org/10.1007/s13157-014-0514-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-014-0514-5