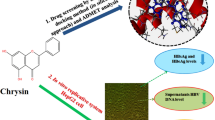
Abstract
Purpose
To label entecavir (ETV) with radioiodine and evaluate its effect on inhibiting hepatitis B virus (HBV) secretion and replication in vitro as well as its biodistribution in BALB/c mice.
Methods
125I-ETV was synthesized via binding a vinyl tributyltin group to ETV and producing electrophilic iodination of the group. Its chemical properties were assessed using traditional methods. Upon intravenous injection of 125I-ETV into BALB/c mice, the radioactivity of the critical organs was detected. In vitro, the anti-HBV activity of 125I-ETV was investigated using HepG2.2.15 cell culture model. Confocal microscopy was used to analyze the cell apoptosis. Culture supernatant samples were used for measuring HBV surface antigen (HBsAg) and HBV e antigen (HBeAg) by enzyme-linked immunosorbent assay. Intracellular HBV pregenomic RNA (pgRNA), DNA, and covalently closed circular DNA (cccDNA) were measured by real-time fluorescence quantitative PCR.
Results
The radiochemical purity of 125I-ETV was greater than 95% after incubation in freshly serum within 48 h. The three highest radioactivities were in the stomach, intestine, and liver after intravenous injection at 0.5 h, 2 h, and 24 h. The confocal fluorescence imaging showed that 125I-ETV did not induce cell apoptosis after treatment for 96 h. 125I-ETV decreases HBsAg and HBeAg secretions as well as intracellular HBV pgRNA, DNA, and cccDNA copies in a dose-dependent manner. Moreover, the anti-HBV activity of 125I-ETV is greater than that of ETV.
Conclusions
The study outcome establishes 125I-ETV as a candidate for anti-HBV. However, it is still in need of further endorsement and optimization by animal model studies before using 125I-ETV to treat chronic HBV disease.









Similar content being viewed by others
Data Availability
Contact the corresponding author for data requests.
References
Younossi ZM, Wong G, Anstee QM, Henry L. The global burden of liver disease. Clin Gastroenterol Hepatol. 2023;21:1978–91.
EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-85.
Butt SRR, Satnarine T, Ratna P, Sarker A, Ramesh AS, Munoz C, et al. A systematic review on current trends in the treatment of chronic hepatitis b to predict disease remission and relapse. Cureus. 2022;14: e32247.
Roade L, Riveiro-Barciela M, Esteban R, Buti M. Long-term efficacy and safety of nucleos(t)ides analogues in patients with chronic hepatitis B. Ther Adv Infect Dis. 2021;8:2049936120985954.
Honkoop P, De Man RA. Entecavir: a potent new antiviral drug for hepatitis B. Expert Opin Investig Drugs. 2003;12:683–8.
Cornberg M, Lok AS, Terrault NA, Zoulim F. Guidance for design and endpoints of clinical trials in chronic hepatitis B - Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference(‡). J Hepatol. 2020;72:539–57.
Lai CL, Wong D, Ip P, Kopaniszen M, Seto WK, Fung J, et al. Reduction of covalently closed circular DNA with long-term nucleos(t)ide analogue treatment in chronic hepatitis B. J Hepatol. 2017;66:275–81.
Malcolm J, Falzone N, Lee BQ, Vallis KA. Targeted radionuclide therapy: New advances for improvement of patient management and response. Cancers (Basel). 2019;11(2):268.
Widel M. Radionuclides in radiation-induced bystander effect; may it share in radionuclide therapy? Neoplasma. 2017;64:641–54.
Gannett PM, Sura TP. An improved synthesis of 8-bromo-2′-deoxyguanosine. Synth Commun. 1993;23:1611–5.
Brancale A, McGuigan C, Andrei G, Snoeck R, De Clercq E, Balzarini J. Bicyclic nucleoside inhibitors of varicella-zoster virus (VZV): the effect of a terminal halogen substitution in the side-chain. Bioorg Med Chem Lett. 2000;10:1215–7.
Cuzzupe AN, Hutton CA, Lilly MJ, Mann RK, McRae KJ, Zammit SC, et al. Total synthesis of the epidermal growth factor inhibitor (-)-reveromycin B. J Org Chem. 2001;66:2382–93.
McBride BJ, Baldwin RM, Kerr JM, Wu J-L. A simple method for the preparation of 123I and 125I labeled iodobenzodiazepines. Int J Radiat Appl Instrum Part A Appl Radiat Isotopes. 1991;42:173–5.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Cai B, Chang S, Tian Y, Zhen S. CRISPR/Cas9 for hepatitis B virus infection treatment. Immun Inflamm Dis. 2023;11: e866.
Yang YC, Yang HC. Recent progress and future prospective in HBV cure by CRISPR/Cas. Viruses. 2021;14(1):4.
Gorsuch CL, Nemec P, Yu M, Xu S, Han D, Smith J, et al. Targeting the hepatitis B cccDNA with a sequence-specific ARCUS nuclease to eliminate hepatitis B virus in vivo. Mol Ther. 2022;30:2909–22.
Jones DE, Vernon CA. Nucleophilic substitution in vinylic halides. Nature. 1955;176:791–2.
Xie L. Inventor A novel iodine-containing antiviral agent. China patent ZL202011151673.9. 2020. https://www.qcc.com/zhuanliDetail/c0c32ae6ab39641ddca64010cb047cad.html.
Innaimo SF, Seifer M, Bisacchi GS, Standring DN, Zahler R, Colonno RJ. Identification of BMS-200475 as a potent and selective inhibitor of hepatitis B virus. Antimicrob Agents Chemother. 1997;41:1444–8.
Ku A, Facca VJ, Cai Z, Reilly RM. Auger electrons for cancer therapy - a review. EJNMMI Radiopharm Chem. 2019;4:27.
Martinez MG, Boyd A, Combe E, Testoni B, Zoulim F. Covalently closed circular DNA: the ultimate therapeutic target for curing HBV infections. J Hepatol. 2021;75:706–17.
Panyutin IV, Sedelnikova OA, Bonner WM, Panyutin IG, Neumann RD. DNA damage produced by 125I-triplex-forming oligonucleotides as a measure of their successful delivery into cell nuclei. Ann N Y Acad Sci. 2005;1058:140–50.
Chen Q, Chen H, Wang W, Liu J, Liu W, Ni P, et al. Glycyrrhetic acid, but not glycyrrhizic acid, strengthened entecavir activity by promoting its subcellular distribution in the liver via efflux inhibition. Eur J Pharm Sci. 2017;106:313–27.
Lai CL, Wong DK, Wong GT, Seto WK, Fung J, Yuen MF. Rebound of HBV DNA after cessation of nucleos/tide analogues in chronic hepatitis B patients with undetectable covalently closed. JHEP Rep. 2020;2: 100112.
Funding
This study was supported by National Natural Science Foundation of China (NSFC), 81701733.
Author information
Authors and Affiliations
Contributions
The study was designed by Liang jun Xie. Material preparation and data collection were performed by Liang jun Xie, M.D., and Mu hua Cheng, Prof. The data analysis was performed by Liang jun Xie, M.D., and Mu hua Cheng, Prof. The first draft of the manuscript was written by Liang jun Xie, M.D., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Mu hua Cheng, Prof., and Liang jun Xie, M.D., declare that they have no competing interests.
Ethics Statement
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Third Affiliated Hospital of Sun Yat-Sen University (No. 00377252).
Consent for Publication
Not applicable.
Disclaimer
The funders had no role in study design, data collection, analysis, and interpretation or preparation of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, M.h., Xie, L.j. Synthesis and Biologic Evaluation of an Iodine-Labeled Entecavir Derivative for Anti-hepatitis B Virus Activity. Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s13139-024-00849-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13139-024-00849-2