Abstract
Purpose
The aim of this study was to investigate whether standard uptake values (SUVs) of pretreatment 18F-FDG PET/CT were the surrogate parameters for predicting the outcomes in locally advanced esophageal squamous cell carcinoma patients treated with definitive chemoradiotherapy.
Materials and Methods
Sixty patients with esophageal squamous cell carcinoma underwent pretreatment 18F-FDG PET/CT and received definitive chemoradiotherapy. 18F-FDG metabolic parameters including SUVmax, SUVmean, SULpeak, total lesion glycolysis (TLG), and metabolic tumor volume (MTV) of primary tumor were calculated. The receiver-operating characteristic (ROC) curve was used to determine the optimal cutoff value of FDG PET/CT-derived parameters that associated with treatment response. Estimating progression-free survival (PFS) and overall survival (OS) was analyzed by using Kaplan–Meier methods. Univariate and multivariate analysis for PFS and OS was performed using Cox regression.
Results
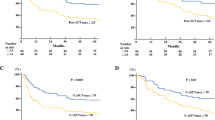
Complete response was achieved in 38.3%. The 4-year OS and PFS rates were 48.6% and 44.4%, respectively. SUVmean with a cutoff value of 6.1 could predict complete response with sensitivity of 69.6%, specificity of 78.4%, and accuracy of 75%. Cox multi-factor regression analyses revealed SUVmean > 6.1 as an independent prognostic factor for OS (HR = 6.74, p = 0.02) and PFS (HR = 6.53, p < 0.001).
Conclusions
Our study suggests that SUVmean of the primary tumor in pretreatment 18F-FDG PET/CT may be used as an independent predictor in esophageal squamous cell carcinoma patients treated with definitive chemoradiotherapy.



Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390(10110):2383–96.
Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371(26):2499–509.
Chen GZ, Zhu HC, Dai WS, Zeng XN, Luo JH, Sun XC. The mechanisms of radioresistance in esophageal squamous cell carcinoma and current strategies in radiosensitivity. J Thorac Dis. 2017;9(3):849–59.
Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17(7):1721–4.
Ding X, Zhang J, Li B, Wang Z, Huang W, Zhou T, et al. A meta-analysis of lymph node metastasis rate for patients with thoracic oesophageal cancer and its implication in delineation of clinical target volume for radiation therapy. Br J Radiol. 2012;85(1019):e1110–9.
Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85–01). Radiation Therapy Oncology Group JAMA. 1999;281(17):1623–7.
Burmeister BH, Dickie G, Smithers BM, Hodge R, Morton K. Thirty-four patients with carcinoma of the cervical esophagus treated with chemoradiation therapy. Arch Otolaryngol Head Neck Surg. 2000;126(2):205–8.
van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84.
Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19(1):68–74.
Kato K, Muro K, Minashi K, Ohtsu A, Ishikura S, Boku N, et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys. 2011;81(3):684–90.
Bruzzi JF, Munden RF, Truong MT, Marom EM, Sabloff BS, Gladish GW, et al. PET/CT of esophageal cancer: its role in clinical management. Radiographics. 2007;27(6):1635–52.
Han S, Kim YJ, Woo S, Suh CH, Lee JJ. Prognostic Value of Volumetric Parameters of Pretreatment 18F-FDG PET/CT in Esophageal Cancer: A Systematic Review and Meta-analysis. Clin Nucl Med. 2018;43(12):887–94.
Rizk NP, Tang L, Adusumilli PS, Bains MS, Akhurst TJ, Ilson D, et al. Predictive value of initial PET-SUVmax in. J Thorac Oncol. 2009;4(7):875–9.
Atsumi K, Nakamura K, Abe K, Hirakawa M, Shioyama Y, Sasaki T, et al. Prediction of outcome with FDG-PET in definitive chemoradiotherapy for esophageal cancer. J Radiat Res. 2013;54(5):890–8.
Suzuki A, Xiao L, Hayashi Y, Macapinlac HA, Welsh J, Lin SH, et al. Prognostic significance of baseline positron emission tomography and importance of clinical complete response in. Cancer. 2011;117(21):4823–33.
Hatt M, Majdoub M, Vallières M, Tixier F, Le Rest CC, Groheux D, et al. 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J Nucl Med : Offi Publ, Soc Nucl Med. 2015; 56(1):38–44.
Koopman D, Jager PL, Slump CH, Knollema S, van Dalen JA. SUV variability in EARL-accredited conventional and digital PET. EJNMMI Res. 2019;9(1):106.
Gopal A, Xi Y, Subramaniam RM, Pinho DF. Intratumoral Metabolic Heterogeneity and Other Quantitative (18)F-FDG PET/CT Parameters for Prognosis Prediction in Esophageal Cancer. Radiol Imaging Cancer 2021; 3 (1):e200022.
Hofheinz F, Li Y, Steffen IG, Lin Q, Lili C, Hua W, et al. Confirmation of the prognostic value of pretherapeutic tumor SUR and MTV in. Eur J Nucl Med Mol Imaging. 2019;46(7):1485–94.
Blom RL, Steenbakkers IR, Lammering G, Vliegen RF, Belgers EJ, de Jonge C, et al. PET/CT-based metabolic tumour volume for response prediction of neoadjuvant chemoradiotherapy in oesophageal carcinoma. Eur J Nucl Med Mol Imaging. 2013;40(10):1500–6.
Vatankulu B, Şanlı Y, Kaytan Sağlam E, Kuyumcu S, Özkan ZG, Yılmaz E, et al. Does Metastatic lymph node SUVmax predict survival in. Mol Imaging Radionuclide Ther. 2015; 24(3):120–7.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015; 42 (2):328–54.
Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging: Off J Inst Clin PET 1999; 2 (3):159–71.
Piessen G, Messager M, Mirabel X, Briez N, Robb WB, Adenis A, et al. Is there a role for surgery for patients with a complete clinical response after chemoradiation for esophageal cancer? An intention-to-treat case-control study. Ann Surg 2013; 258 (5):793–9; discussion 99–800.
Chen AP, Setser A, Anadkat MJ, Cotliar J, Olsen EA, Garden BC, et al. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol 2012; 67 (5):1025–39.
Milenović Ž. Application of Mann-Whitney U test in research of professional training of primary school teachers. Metodički obzori: časopis za odgojno-obrazovnu teoriju i praksu. 2011;6(11):73–9.
Grabowski B. "P < 0.05" Might not mean what you think: American statistical association clarifies P values. J Natl Cancer Inst 2016; 108 (8).
Anderluh F, Toplak M, Velenik V, Oblak I, Ermenc AS, Peressutti AJ, et al. Definitive radiochemotherapy in esophageal cancer - a single institution experience %J Radiol Oncol. 2019; 53 (4):480–87.
Versteijne E, van Laarhoven HWM, van Hooft JE, van Os RM, Geijsen ED, van Berge Henegouwen MI, et al. Definitive chemoradiation for patients with inoperable and/or unresectable esophageal cancer: locoregional recurrence pattern. Dis Esophagus. 2015;28(5):453–9.
Li C, Lin JW, Yeh HL, Chuang CY, Chen CC. Good prediction of treatment responses to neoadjuvant chemoradiotherapy for esophageal cancer based on preoperative inflammatory status and tumor glucose metabolism. Sci Rep. 2021;11(1):11626.
Takahashi N, Umezawa R, Takanami K, Yamamoto T, Ishikawa Y, Kozumi M, et al. Whole-body total lesion glycolysis is an independent predictor in. Radiother Oncol. 2018;129(1):161–5.
Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, et al. Chemoradiation with and without surgery in. J Clin Oncol : Off J Am Soc Clin Oncol. 2005; 23(10):2310–7.
Lu J, Sun XD, Yang X, Tang XY, Qin Q, Zhu HC, et al. Impact of PET/CT on radiation treatment in. Crit Rev Oncol Hematol. 2016;107:128–37.
Hatt M, Visvikis D, Pradier O, Cheze-le RC. Baseline (1)(8)F-FDG PET image-derived parameters for therapy response prediction in oesophageal cancer. Eur J Nucl Med Mol Imaging. 2011;38(9):1595–606.
Nakajo M, Jinguji M, Nakabeppu Y, Nakajo M, Higashi R, Fukukura Y, et al. Texture analysis of (18)F-FDG PET/CT to predict tumour response and prognosis of patients with esophageal cancer treated by chemoradiotherapy. Eur J Nucl Med Mol Imaging. 2017;44(2):206–14.
Cervino AR, Evangelista L, Alfieri R, Castoro C, Sileni VC, Pomerri F, et al. Positron emission tomography/computed tomography and esophageal cancer in the clinical practice: How does it affect the prognosis? J Cancer Res Ther. 2012;8(4):619–25.
Nahmias C, Wahl LM. Reproducibility of standardized uptake value measurements determined by 18F-FDG PET in malignant tumors. J Nucl Med. 2008;49(11):1804–8.
Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol. 2010;195(2):310–20.
Mantziari S, Pomoni A, Prior JO, Winiker M, Allemann P, Demartines N, et al. (18)F- FDG PET/CT-derived parameters predict clinical stage and prognosis of esophageal cancer. BMC Med Imaging. 2020;20(1):7.
Acknowledgements
We thank all staffs in the Nuclear Medicine, Radiation Oncology & Radiosurgery Department for collecting the data and Ms Le Kieu Minh, Mr Pham Xuan Hung and Mr Glenn Sy for the technical support.
Author information
Authors and Affiliations
Contributions
Basic study idea was made by M.H.S; L.N.H and N.D.C conducted patient management and procedures. Data were curated by N.D.C and B.Q.B., analyzed by M.H.S and N.D.C, and validated by L.N.H. Draft of manuscript was written by L.N.H. M.H.S. and N.D.C and reviewed by all authors. M.H.S edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
Le Ngoc Ha, Nguyen Dinh Chau, Bui Quang Bieu, and Mai Hong Son declare that they have no conflict of interest.
Ethics Approval and Consent to Participate
This study was approved by the institutional review board of Central Military Hospital 108 (approval no. 165/QD-V108).
Consent for Publication
As the procedures involved were standard of care, the committee agreed to waive the need for written informed consent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ha, L.N., Chau, N.D., Bieu, B.Q. et al. Pretreatment 18F-FDG PET/CT-Derived Parameters in Predicting Clinical Outcomes of Locally Advanced Upper Third Esophageal Squamous Cell Carcinoma After Definitive Chemoradiation Therapy. Nucl Med Mol Imaging 56, 181–187 (2022). https://doi.org/10.1007/s13139-022-00751-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-022-00751-9