Abstract
Purpose
To compare 131I-therapy outcomes in high turnover and normal turnover Graves’ disease patients and predict optimal first 131I activity for high turnover patients.
Methods
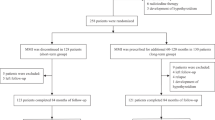
Retrospective cohort design (1:2) validated by propensity score analysis. Cohort 1, high turnover (2-h RAIU/24-h RAIU ≥ 1), n = 104, and cohort 2, normal turnover (ratio < 1), n = 208, patients were compared for post 131I outcome. The cure was defined as a combined euthyroid and stable hypothyroid state following 131I treatment. Logistic regression analysis was used for identifying prognostic factors. The propensity score was applied; 77 matched pairs (1:1 ratio) of high and normal turnover patients were selected as a validation set.
Results
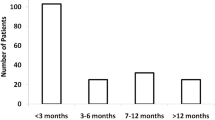
First 131I cure rates of 28% in high turnover and 66% in normal turnover groups (p = 0.001) were noted. The therapy cycles (median, 2 vs. 1) and cumulative 131I activity (median, 15 vs. 7 mCi) were required to cure hyperthyroidism in cohort 1 and cohort 2, respectively. Age (> 44 years), higher grade of goitre, and 2-h RAIU (> 37%) were associated with 131I therapy failure. The high turnover patients needed a factor of 1.5–2 times more 131I activity to achieve a similar cure rate compared to the normal turnover patients. The first-dose cure rate was 31% vs. 60% by propensity score analysis (n = 154), no way different (28% vs.66%) from the whole group of 312 patients.
Conclusion
High turnover Graves’ disease patients, if administered standard 131I activity, the outcomes shall be poor. To improve the success rate, 131I activity should be increased by 1.5 to 2 times in the high turnover patients.


Similar content being viewed by others
Data Availability
Original raw data is available on request to corresponding author.
References
Törring O, Tallstedt L, Wallin G, Lundell G, Ljunggren JG, Taube A, et al. Graves’ hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine-a prospective, randomized study. Thyroid Study Group. J Clin Endocrinol Metab. 1996;81:2986–93.
Abraham P, Avenell A, McGeoch SC, Clark LF, Bevan JS. Antithyroid drug regimen for treating Graves’ hyperthyroidism. Cochrane Database Syst Rev. 2010;2010:CD003420.
Wartofsky L, Glinoer D, Solomon B, Nagataki S, Lagasse R, Nagayama Y, et al. Differences and similarities in the diagnosis and treatment of Graves’ disease in Europe, Japan, and the United States. Thyroid. 1991;1:129–35.
de Rooij A, Vandenbroucke JP, Smit JW, Stokkel MP, Dekkers OM. Clinical outcomes after estimated versus calculated activity of radioiodine for the treatment of hyperthyroidism: systematic review and meta-analysis. Eur J Endocrinol. 2009;161:771–7.
Jaiswal AK, Bal C, Damle NA, Ballal S, Goswami R, Hari S, et al. Comparison of clinical outcome after a fixed-dose versus dosimetry-based radioiodine treatment of Graves’ disease: results of a randomized controlled trial in Indian population. Indian J Endocrinol Metab. 2014;18:648–54.
Jarlov AE, Hegedus L, Kristensen LO, Nygaard B, Hansen JM. Is the calculation of the dose in radioiodine therapy of hyperthyroidism worthwhile? Clin Endocrinol. 1995;43:325–9.
Peters H, Fischer C, Bogner U, Reiners C, Schleusener H. Radioiodine therapy of Graves’ hyperthyroidism: standard vs calculated 131iodine activity. Results from a prospective, randomized, multicentre study. Eur J Clin Investig. 1995;25:186–93.
Peters H, Fischer C, Bogner U, Reiners C, Schleusener H. Reduction in thyroid volume after radioiodine therapy of Graves’ hyperthyroidism: results of a prospective, randomized, multicentre study. Eur J Clin Investig. 1996;26:59–63.
Stokkel MP, HandkiewiczJunak D, Lassmann M, Dietlein M, Lusteret M. EANM procedure guidelines for therapy of benign thyroid disease. Eur J Nucl Med Mol Imaging. 2010;37:2218–28.
Marinelli LD, Quimby EH, Hine GJ. Dosage determination with radioactive isotopes; practical considerations in therapy and protection. Am J Roentgenol Radium Ther. 1948;59:260–81.
Leslie WD, Ward L, Salamon EA, Ludwig S, Rowe RC, Cowden EA. A randomized comparison of radioiodine doses in Graves' hyperthyroidism. J Clin Endocrinol Metab. 2003;88:978–83.
Berg GEB, Michanek AMK, Holmberg ECV, Fink M. Iodine-131 treatment of hyperthyroidism: the significance of effective half-life measurements. J Nucl Med. 1996;37:228–32.
Aktay R, Rezai K, Seabold JE, Bar RS, Kirchner PT. Four- to twenty-four-hour uptake ratio: an index of rapid iodine-131 turnover in hyperthyroidism. J Nucl Med. 1996;37:1815–9.
Clerc J, Izembart M, Dagousset F, Jaïs JP, Heshmati HM, Chevalier A, et al. Influence of dose selection on absorbed dose profiles in radioiodine treatment of diffuse toxic goitres in patients receiving or not receiving carbimazole. J Nucl Med. 1993;34:387–93.
Kobe C, Eschner W, Wild M, Rahlff I, Sudbrock F, Schmidt M, et al. Radioiodine therapy of benign thyroid disorders: what are the effective thyroidal half-life and uptake of 131I? Nucl Med Commun. 2010;31:201–5.
Becker DV, Hurley JR. The impact of technology on clinical practice in Graves' disease. Mayo Clin Proc. 1972;47:835–47.
Harbert JC. Radioiodine therapy of hyperthyroidism. In: Harbert JC, Eckelman WC, Neumann RD. eds. Nuclear medicine diagnosis and therapy. New York: Thieme Medical Publishing Inc.: 1996:951–973.
de Jong JA, Verkooijen HM, Valk GD, Zelissen PM, de Keizer B. High failure rates after (131)I therapy in Graves hyperthyroidism patients with large thyroid volumes, high iodine uptake, and high iodine turnover. Clin Nucl Med. 2013;38:401–6.
Sekulic V, Rajic M, Vlajkovic M, Ilić S, Stević M, Kojić M. The effect of short-term treatment with lithium carbonate on the outcome of radioiodine therapy in patients with long-lasting Graves’ hyperthyroidism. Ann Nucl Med. 2017;31:744–51.
Thamcharoenvipas S, Kerr SJ, Tepmongkol S. Finding the best effective way of treatment for rapid I-131 turnover Graves’ disease patients: a randomized clinical trial. Medicine (Baltimore). 2019;98:15573.
Bal CS, Kumar A, Pandey RM. A randomized controlled trial to evaluate the adjuvant effect of lithium on radioiodine treatment of hyperthyroidism. Thyroid. 2002;12:399–405.
Kalinyak JE, McDougall IR. How should the dose of iodine-131 be determined in the treatment of Graves’ hyperthyroidism? J Clin Endocrinol Metab. 2003;88:975–7.
Peters H, Fischer C, Bogner U, Reiners C, Schleusener H. Treatment of Graves’ hyperthyroidism with radioiodine: results of a prospective randomized study. Thyroid. 1997;7:247–51.
Van Isselt JW, de Klerk JMH, Koppeschaar HPF, Koppeschaar HP, Van Rijk PP. Iodine-131 uptake and turnover rate vary over short intervals in Graves’ disease. Nucl Med Commun. 2000;21:609–16.
Ballal S, Soundararajan R, Bal C. Re-establishment of normal radioactive iodine uptake reference range in the era of universal salt iodization in the Indian population. Indian J Med Res. 2017;145:358–64.
Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35:121–6.
Fleiss JL. Statistical methods for rates and proportions. 2nd ed. New York: Wiley; 1981. p. 45.
Braga M, Walpert N, Burch HB, Solomon BL, Cooper DS. The effect of methimazole on cure rates after radioiodine treatment for Graves’ hyperthyroidism: a randomized clinical trial. Thyroid. 2002;12:135–9.
World Health Organization, International Council for Control of Iodine Deficiency Disorders & United Nations Children's Fund (UNICEF). Indicators for assessing iodine deficiency disorders and their control through salt iodization. World Health Organization; 1994. https://apps.who.int/iris/handle/10665/70715.
Zhang R, Tan J, Wang R, Zhang G, Jia Q, Meng Z, et al. Analysis of risk factors of rapid thyroidal radioiodine-131 turnover in Graves’ disease patients. Sci Rep. 2017;7:8301.
Luo Z, Gardiner JC, Bradley CJ. Applying propensity score methods in medical research: pitfalls and prospects. Med Care Res Rev. 2010;67:528–54.
Gum PA, Thamilarasan M, Watanabe J, Blackstone EH, Lauer MS. Aspirin use and all-cause mortality among patients being evaluated for known or suspected coronary artery disease: a propensity analysis. JAMA. 2001;286:1187–94.
Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–77.
Green M, Fisher M, Miller H, Wilson GM. Blood radiation dose after I therapy of thyrotoxicosis. Calculations with reference to leukemia. Br Med J. 1961;2:210–5.
Rail JE, Sonenberg MS, Robbins J, Lazerson R, Rawson RW. The blood level as a guide to therapy with radioiodine. J Clin Endocrinol Metab. 1953;13:1369–77.
Pinyowatanasilp P, Uaratanawong S. Therapy dose calculation in hyperthyroidism using the 3-hour early i-131 uptake measurements. Vajira Med J. 2005;49:147–52.
Morris LF, Waxman AD, Braunstein GD. Accuracy considerations when using early (four- or six-hour) radioactive iodine uptake to predict twenty-four-hour values for radioactive iodine dosage in the treatment of Graves’ disease. Thyroid. 2000;10:779–87.
Baczyk M, Junik R, Ziemnicka K, Sowiński J. Iodine prophylaxis intensification. Influence on radioiodine uptake and activity of 131I used in the treatment of hyperthyroid patients with Graves' disease. Nuklearmedizin. 2005;44:197–9.
Zimmermann MB. Iodine deficiency and endemic cretinism. In: Braverman LE, Cooper DS, editors. Werner & Ingbar’s the thyroid a fundamental and clinical text. Philadelphia: Lippincott Williams & Wilkins; 2013. p. 217–41.
Cunnien AJ, Hay ID, Gorman CA, Offord KP, Scanlon PW. Radioiodine-induced hypothyroidism in Graves’ disease: factors associated with the increasing incidence. J Nucl Med. 1982;23:978–83.
Andrade VA, Gross JL, Maia AL. The effect of methimazole pretreatment on the efficacy of radioactive iodine therapy in Graves’ hyperthyroidism: one-year follow-up of a prospective, randomized study. J Clin Endocrinol Metab. 2001;86:3488–93.
Allahabadia A, Daykin J, Holder RL, Sheppard MC, Gough SC, Franklyn JA. Age and gender predict the outcome of treatment for Graves' hyperthyroidism. J Clin Endocrinol Metab. 2000;85:1038–42.
Bonnema SJ, Bennedbaek FN, Veje A, Marving J, Hegedüs L. Propylthiouracil before 131I therapy of hyperthyroid diseases: effect on cure rate evaluated by a randomized clinical trial. J Clin Endocrinol Metab. 2004;89:4439–44.
DeGroot LJ, Stanbury JB. Graves’ disease: diagnosis and treatment. In: DeGroot LJ, Stanbury JB, editors. The thyroid and its diseases. 4th ed. New York: John Wiley & Sons; 1975. p. 314–67.
De Bruin TWA, Croon CDL, de Klerk JMH, van Isselt JW. Standardized radioiodine therapy in Graves’ disease: the persistent effect of thyroid weight and radioiodine uptake on the outcome. J Intern Med. 1994;236:507–13.
Damle N, Bal C, Kumar P, Reddy R, Virkar D. The predictive role of 24h RAIU with respect to the outcome of low fixed-dose radioiodine therapy in patients with diffuse toxic goiter. Hormones (Athens). 2012;11:451–7.
Kristoffersen US, Hesse B, Rasmussen AK, Kjaer A. Radioiodine therapy in hyperthyroid disease: poorer outcome in patients with high 24 hours radioiodine uptake. Clin Physiol Funct Imaging. 2006;26:167–70.
van Isselt JW, Broekhuizen-de Gast HS. The radioiodine turnover rate as a determinant of radioiodine treatment outcome in Graves’ disease. Hell J Nucl Med. 2010;13:2–5.
Marcocci C, Gianchecchi D, Masini I, Golia F, Ceccarelli C, Bracci E, et al. A reappraisal of the role of methimazole and other factors on the efficacy and outcome of radioiodine therapy of Graves’ hyperthyroidism. J Endocrinol Investig. 1990;13:513–20.
Allahabadia A, Daykin J, Shappard MC, Gough SC, Franklyn JA. Radioiodine treatment of hyperthyroidism- prognostic factors and outcome. J Clin Endocrinol Metab. 2001;86:3611–7.
Metso S, Jaatinen P, Huhtala H, Luukkaala T, Oksala H, Salmi J. A long-term follow-up study of radioiodine treatment of hyperthyroidism. Clin Endocrinol. 2004;61:641–8.
Nygaard B, Hegedü SL, Gervil M, Hjalgrim H, Hansen BM, Søe-Jensen P, et al. Influence of compensated radioiodine therapy on thyroid volume and incidence of hypothyroidism in Graves’ disease. J Intern Med. 1995;238:491–7.
Acknowledgements
We want to thank Dr. M.A Khan and Mr. Hem Chandra Sati, from Department of Biostatistics, AIIMS, New Delhi, for their help related to statistical analysis.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Saurabh Arora and Chandrasekhar Bal. The first draft of the manuscript was written by Saurabh Arora, and Chandrasekhar Bal commented on previous versions of the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Saurabh Arora and Chandrasekhar Bal declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration as revised in 2013 and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Informed Consent
The institute ethics committee (Human studies), All India institute of Medical Sciences, New Delhi, approved this retrospective study (ref no: IECPG-598/24.10.2019) and the requirement to obtain informed consent was waived.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 145 kb)
Rights and permissions
About this article
Cite this article
Arora, S., Bal, C. Is There Any Need for Adjusting 131I Activity for the Treatment of High Turnover Graves’ Disease Compared to Normal Turnover Patients? Results from a Retrospective Cohort Study Validated by Propensity Score Analysis. Nucl Med Mol Imaging 55, 15–26 (2021). https://doi.org/10.1007/s13139-020-00674-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-020-00674-3