Abstract
Purpose
Intracranial administration of lipopolysaccharide (LPS) is known to elicit a rapid innate immune response, activate glial cells in the brain, and induce depression-like behavior. However, no study has focused on the changes in glial cells induced by intraperitoneal injection of LPS in vivo.
Methods
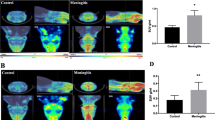
Ten adult male Fischer F344 rats underwent [11C]PK11195 PET before and 2 days after intraperitoneal injection of LPS to evaluate the changes in glial cells. The difference in standardized uptake values (SUV) of [11C]PK11195 between before and after injection was determined.
Results
There was a cluster of brain regions that showed significant reductions in SUV. This cluster included the bilateral striata and bilateral frontal regions, especially the somatosensory areas.
Conclusions
Changes in activity of glial cells induced by the intraperitoneal injection of LPS were detected in vivo by [11C]PK11195 PET. Intraperitoneal injection of LPS is known to induce depression, and further studies with [11C]PK11195 PET would clarify the relationships between neuroinflammation and depression.

Similar content being viewed by others
References
Kunugi H, Hori H, Ogawa S. Biochemical markers subtyping major depressive disorder. Psychiatry Clin Neurosci. 2015;69:597–608.
Pollak Y, Yirmiya R. Cytokine-induced changes in mood and behaviour: implications for 'depression due to a general medical condition', immunotherapy and antidepressive treatment. Int J Neuropsychopharmacol. 2002;5:389–99.
Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–52.
Martin SA, Dantzer R, Kelley KW, Woods JA. Voluntary wheel running does not affect lipopolysaccharide-induced depressive-like behavior in young adult and aged mice. Neuroimmunomodulation. 2014;21:52–63.
Sulakhiya K, Kumar P, Jangra A, Dwivedi S, Hazarika NK, Baruah CC, et al. Honokiol abrogates lipopolysaccharide-induced depressive like behavior by impeding neuroinflammation and oxido-nitrosative stress in mice. Eur J Pharmacol. 2014;744:124–31.
Bay-Richter C, Janelidze S, Hallberg L, Brundin L. Changes in behaviour and cytokine expression upon a peripheral immune challenge. Behav Brain Res. 2011;222:193–9.
De La Garza R II, Fabrizio KR, Radoi G-E, Vlad T, Asnis GM. The non-steroidal anti-inflammatory drug diclofenac sodium attenuates lipopolysaccharide-induced alterations to reward behavior and corticosterone release. Behav Brain Res. 2004;149:77–85.
Engeland CG, Kavaliers M, Ossenkopp KP. Sex differences in the effects of muramyl dipeptide and lipopolysaccharide on locomotor activity and the development of behavioral tolerance in rats. Pharmacol Biochem Behav. 2003;74:433–47.
Moraes MM, Galvão MC, Cabral D, Coelho CP, Queiroz-Hazarbassanov N, Martins MF, et al. Propentofylline prevents sickness behavior and depressive-like behavior induced by lipopolysaccharide in rats via neuroinflammatory pathway. PLoS One. 2017;12:e0169446.
Tonelli LH, Holmes A, Postolache TT. Intranasal immune challenge induces sex-dependent depressive-like behavior and cytokine expression in the brain. Neuropsychopharmacology. 2008;33:1038–48.
Sasayama D, Hattori K, Wakabayashi C, Teraishi T, Hori H, Ota M, et al. Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J Psychiatr Res. 2013;47:401–6.
Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12:114.
Ng YK, Ling EA. Induction of major histocompatibility class II antigen on microglial cells in postnatal and adult rats following intraperitoneal injections of lipopolysaccharide. Neurosci Res. 1997;28:111–8.
Cazareth J, Guyon A, Heurteaux C, Chabry J, Petit-Paitel A. Molecular and cellular neuroinflammatory status of mouse brain after systemic lipopolysaccharide challenge: importance of CCR2/CCL2 signaling. J Neuroinflammation. 2014;11:132.
Wu SY, Chen YW, Tsai SF, Wu SN, Shih YH, Jiang-Shieh YF, et al. Estrogen ameliorates microglial activation by inhibiting the Kir2.1 inward-rectifier K(+) channel. Sci Rep. 2016;6:22864.
Hannestad J, DellaGioia N, Gallezot JD, Lim K, Nabulsi N, Esterlis I, et al. The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [11C]PBR28 PET study. Brain Behav Immun. 2013;33:131–8.
Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiat. 2015;72:268–75.
Su L, Faluyi YO, Hong YT, Fryer TD, Mak E, Gabel S, et al. Neuroinflammatory and morphological changes in late-life depression: the NIMROD study. Br J Psychiatry. 2016;209:525–6.
Haarman BC, Burger H, Doorduin J, Renken RJ, Sibeijn-Kuiper AJ, Marsman JB, et al. Volume, metabolites and neuroinflammation of the hippocampus in bipolar disorder – a combined magnetic resonance imaging and positron emission tomography study. Brain Behav Immun. 2016;56:21–33.
van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry. 2008;64:820–2.
Dickens AM, Vainio S, Marjamäki P, Johansson J, Lehtiniemi P, Rokka J, et al. Detection of microglial activation in an acute model of neuroinflammation using PET and radiotracers 11C-(R)-PK11195 and 18F-GE-180. J Nucl Med. 2014;55:466–72.
Sridharan S, Lepelletier FX, Trigg W, Banister S, Reekie T, Kassiou M, et al. Comparative evaluation of three TSPO PET radiotracers in a LPS-induced model of mild neuroinflammation in rats. Mol Imaging Biol. 2017;19:77–89.
Shah F, Hume SP, Pike VW, Ashworth S, McDermott J. Synthesis of the enantiomers of [N-methyl-11C]PK 11195 and comparison of their behaviours as radioligands for PK binding sites in rats. Nucl Med Biol. 1994;21:573–81.
Mizuta T, Kitamura K, Iwata H, Yamagishi Y, Ohtani A, Tanaka K, et al. Performance evaluation of a high-sensitivity large-aperture small-animal PET scanner: ClairvivoPET. Ann Nucl Med. 2008;22:447–55.
Tanaka E, Kudo H. Subset-dependent relaxation in block-iterative algorithms for image reconstruction in emission tomography. Phys Med Biol. 2003;48:1405–22.
de Paula FD, de Vries EF, Sijbesma JW, Buchpiguel CA, Dierckx RA, Copray SC. PET imaging of glucose metabolism, neuroinflammation and demyelination in the lysolecithin rat model for multiple sclerosis. Mult Scler. 2014;20:1443–52.
Ito S. Visceral region in the rat primary somatosensory cortex identified by vagal evoked potential. J Comp Neurol. 2002;444:10–24.
Schweighöfer H, Rummel C, Roth J, Rosengarten B. Modulatory effects of vagal stimulation on neurophysiological parameters and the cellular immune response in the rat brain during systemic inflammation. Intensive Care Med Exp. 2016;4:19.
Wolff S, Klatt S, Wolff JC, Wilhelm J, Fink L, Kaps M, et al. Endotoxin-induced gene expression differences in the brain and effects of iNOS inhibition and norepinephrine. Intensive Care Med. 2009;35:730–9.
Kropholler MA, Boellaard R, van Berckel BN, Schuitemaker A, Kloet RW, Lubberink MJ, et al. Evaluation of reference regions for (R)-[11C]-PK11195 studies in Alzheimer’s disease and mild cognitive impairment. J Cereb Blood Flow Metab. 2007;27:1965–74.
Nakatomi Y, Mizuno K, Ishii A, Wada Y, Tanaka M, Tazawa S, et al. Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: an 11C-(R)-PK11195 PET study. J Nucl Med. 2014;55:945–50.
Vas A, Shchukin Y, Karrenbauer VD, Cselényi Z, Kostulas K, Hillert J, et al. Functional neuroimaging in multiple sclerosis with radiolabelled glia markers: preliminary comparative PET studies with [11C]vinpocetine and [11C]PK11195 in patients. J Neurol Sci. 2008;264:9–17.
Koyama M, Kawashima R, Ito H, Ono S, Sato K, Goto R, et al. SPECT imaging of normal subjects with technetium-99m-HMPAO and technetium-99m-ECD. J Nucl Med. 1997;38:587–92.
Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation. 2011;8:94.
Van Otterloo ES, Miguel-Hidalgo JJ, Stockmeier CA, Rajkowska G. Microglia immunoreactivity is unchanged in the white matter of orbitofrontal cortex in elderly depressed patients. Program no. 9152. Neuroscience 2005 Abstracts. Washington DC. Society for Neuroscience. 2005
Czeh M, Gressens P, Kaindl AM. The yin and yang of microglia. Dev Neurosci. 2011;33:199–209.
Wee Yong V. Inflammation in neurological disorders: a help or a hindrance? Neuroscientist. 2010;16:408–20.
Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–75.
Koizumi S, Ohsawa K, Inoue K, Kohsaka S. Purinergic receptors in microglia: functional modal shifts of microglia mediated by P2 and P1 receptors. Glia. 2013;61:47–54.
Gavish M, Bachman I, Shoukrun R, Katz Y, Veenman L, Weisinger G, et al. Enigma of the peripheral benzodiazepine receptor. Pharmacol Rev. 1999;51:629–50.
Acknowledgements
The authors thank Mr. Makoto Funasaka for his expert technical assistance with the PET experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
Miho Ota was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (C) (16 K10234). Jun Ogura, Shintaro Ogawa, Koichi Kato, Hiroshi Matsuda, and Hiroshi Kunugi declare that they have no conflicts of interest.
Ethical Approval
All experimental procedures were in accordance with the guidelines of the United State’s National Institutes of Health (1996) (http://oacu.od.nih.gov/regs/guide/guide.pdf) and were approved by the Ethics Review Committee for Animal Experimentation at the National Institute of Neuroscience, National Center of Neurology and Psychiatry, Japan, and performed with every effort to minimize the number of animals and their suffering.
Informed Consent
The Ethics Review Committee for Animal Experimentation of our institution approved this study, and the requirement to obtain informed consent was waived.
Rights and permissions
About this article
Cite this article
Ota, M., Ogura, J., Ogawa, S. et al. A Single Intraperitoneal Injection of Endotoxin Changes Glial Cells in Rats as Revealed by Positron Emission Tomography Using [11C]PK11195. Nucl Med Mol Imaging 52, 224–228 (2018). https://doi.org/10.1007/s13139-017-0510-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-017-0510-9