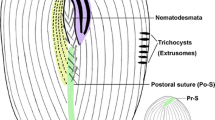
Abstract
Paramecium is one of the best known and most intensely studied ciliate genera. It currently comprises 18 morphospecies including the P. aurelia complex of 15 sibling species. Here, we describe the new morphospecies Paramecium buetschlii sp. nov. from a freshwater pool in Norway, featuring unusual combinations of morphological characters and a high genetic diversity relative to other congeners. Three further investigated Paramecium spp. from Germany, Hungary, and Brazil are treated as cryptic species, because they are difficult to discriminate from other members of the genus relying on morphological criteria only. However, DNA-based taxonomic markers (18S-rDNA and mitochondrial COI) clearly indicate they are separate species. Due to the lack of an appropriate systematic term within the International Code of Zoological Nomenclature for distinguishing cryptic from valid biological species, we propose the provisional status Eucandidatus as a component of the taxonomic name when describing new but cryptic eukaryotes. Based on our data, we postulate that even within Europe there is a higher biodiversity within this common ciliate group that is heavily used in the classroom. By uncovering potentially distinct species that have been classified under the same species names, our molecular analyses further suggest a higher current stock diversity in Paramecium than previously thought. We also would like to emphasize that under-sampling is another major issue in estimating protist diversity. Future large-scale studies based on extensive sampling not only in exotic and remote regions, but also in less frequently sampled areas, will therefore likely improve our understanding of species richness and diversity.










Similar content being viewed by others
References
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic Local Alignment Search Tool. Journal of Molecular Biology, 215(3), 403–410.
Aufderheide, K. J., Daggett, P. M., & Nerad, T. A. (1983). Paramecium sonneborni n. sp., a new member of the Paramecium aurelia species-complex. Journal of Protozoology, 30, 128–131.
Baker, A. J., Daugherty, C. H., Colbourne, R., & Mclennan, J. L. (1995). Flightless brown kiwis of New Zealand possess extremely subdivided population structure and cryptic species like small mammals. Proceedings of the National Academy of Sciences of the United States of America, 92(18), 8254–8258.
Barth, D., Krenek, S., Fokin, S. I., & Berendonk, T. U. (2006). Intraspecific genetic variation in Paramecium revealed by mitochondrial cytochrome c oxidase I sequences. Journal of Eukaryotic Microbiology, 53(1), 20–25.
Beale, G. H., & Preer, J. R. J. (2008). Paramecium: genetics and epigenetics. Boca Raton: CRC.
Bickford, D., Lohman, D. J., Sodhi, N. S., Ng, P. K. L., Meier, R., Winker, K., et al. (2007). Cryptic species as a window on diversity and conservation. Trends in Ecology & Evolution, 22(3), 148–155.
Boscaro, V., Fokin, S. I., Verni, F., & Petroni, G. (2012). Survey of Paramecium duboscqui using three markers and assessment of the molecular variability in the genus Paramecium. Molecular Phylogenetics and Evolution, 65(3), 1004–1013.
Bouin, P. A. (1897). Etudes sur l’évolution normale et l’involution du tube seminifère. Archives d’Anatomie Microscopique et de Morphologie Expérimentale, 1, 225–339.
Buosi, P. R. B., Cabral, A. F., Simao, T. L. L., Utz, L. R. P., & Velho, L. F. M. (2014). Multiple lines of evidence shed light on the occurrence of Paramecium (Ciliophora, Oligohymenophorea) in bromeliad tank water. Journal of Eukaryotic Microbiology, 61(1), 2–10.
Capella-Gutiérrez, S., Silla-Martínez, J. M., & Gabaldón, T. (2009). trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics.
Chatton, E., & Brachon, S. (1933). Sur une paramécie à deux races: Paramœcium dubosqui n. sp. Comptes Rendus Societe Biologie, 114, 4.
Claparède, R.-É., & Lachmann, J. (1858). Études sur les infusoires et les rhizopodes;. Genève: Vaney, H. Georg, pp. 1–482.
Cook, L. G., Edwards, R. D., Crisp, M. D., & Hardy, N. B. (2010). Need morphology always be required for new species descriptions? Invertebrate Systematics, 24(3), 322–326.
Corliss, J. O. (1953). Silver impregnation of ciliated protozoa by the Chatton-Lwoff technic. Stain Technology, 28(2), 97–100.
Darriba, D., Taboada, G. L., Doallo, R., & Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9(8), 772.
de Leon, G. P. P., & Nadler, S. A. (2010). What we don’t recognize can hurt us: a plea for awareness about cryptic species. Journal of Parasitology, 96(2), 453–464.
Diller, W. F., & Earl, P. R. (1958). Paramecium jenningsi n. sp. Journal of Protozoology, 5(2), 155–158.
Dragesco, J. (1970). Ciliés libres du Cameroun. Yaoundé: Ann. Fac. Sci. Univ. Fed. Cameroun.
Dragesco, J. (1972). Free living ciliates from Uganda. Annal Faculty Science Cameroun, 9, 87–126.
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32(5), 1792–1797.
Ehrenberg, C. G. (1838). Die Infusionsthierchen als vollkommene Organismen. Ein Blick in das tiefere organische Leben der Natur. Leipzig: L. Voss.
Fehlauer-Ale, K. H., Mackie, J. A., Lim-Fong, G. E., Ale, E., Pie, M. R., & Waeschenbach, A. (2014). Cryptic species in the cosmopolitan Bugula neritina complex (Bryozoa, Cheilostomata). Zoologica Scripta, 43(2), 193–205.
Fenchel, T., & Finlay, B. J. (2006). The diversity of microbes: resurgence of the phenotype. Philosophical Transactions of the Royal Society, B: Biological Sciences, 361(1475), 1965–1973.
Focke, G .W. (1836). Ueber einige Organisationsverhältnisse bei polygastrischen Infusorien und Räderthieren. Oken. Isis, 10, 785–787.
Foissner, W. (1991). Basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa. European Journal of Protistology, 27(4), 313–330.
Foissner, W., Berger, H., & Schaumburg, J. (1999). Identification and ecology of limnetic plankton ciliates. München: Bayerisches Landesamt für Wasserwirtschaft, 793 pp.
Fokin, S. I. (2010/11). Paramecium genus: biodiversity, some morphological features and the key to the main morphospecies discrimination. Protistology, 6(4), 227–235.
Fokin, S. I., & Chivilev, S. M. (1999). Brackish water Paramecium species and Paramecium polycaryum. Morphometrical analysis and some biological peculiarities. Acta Protozoologica, 38(2), 105–117.
Fokin, S. I., & Chivilev, S. M. (2000). Paramecium. Morphometric analysis and taxonomy. Acta Protozoologica, 39(1), 1–14.
Fokin, S. I., Stoeck, T., & Schmidt, H. J. (1999a). Paramecium duboscqui Chatton & Brachon, 1933. Distribution, ecology and taxonomy. European Journal of Protistology, 35(2), 161–167.
Fokin, S. I., Stoeck, T., & Schmidt, H. J. (1999b). Rediscovery of Paramecium nephridiatum Gelei, 1925 and its characteristics. Journal of Eukaryotic Microbiology, 46(4), 416–426.
Fokin, S. I., Przybos, E., & Chivilev, S. M. (2001). Nuclear reorganization variety in Paramecium (Ciliophora: Peniculida) and its possible evolution. Acta Protozoologica, 40(4), 249–261.
Fokin, S. I., Przybos, E., Chivilev, S. M., Beier, C. L., Horn, M., Skotarczak, B., et al. (2004). Morphological and molecular investigations of Paramecium schewiakoffi sp. nov. (Ciliophora, Oligohymenophorea) and current status of distribution and taxonomy of Paramecium spp. European Journal of Protistology, 40(3), 225–243.
Funk, W. C., Caminer, M., & Ron, S. R. (2011). High levels of cryptic species diversity uncovered in Amazonian frogs. Proceedings of the Royal Society B: Biological Sciences.
Gelei, J. (1925). Uj Paramecium szeged kornyekerol. Paramecium nephridiatum nov. sp. Allat Kozlony Zoology Mitteilungen, 22, 121–162 (In Hungarian with German summary).
Gelei, J. (1938). Beitrage zur Ciliatenfauna der Umgebung von Szeged. VII. Paramecium nephridiatum. Archiv fuer Protistenkunde, 91, 343–356.
Goldstein, P. Z., & DeSalle, R. (2011). Integrating DNA barcode data and taxonomic practice: determination, discovery, and description. Bioessays, 33(2), 135–147.
Görtz, H. D. (Ed.). (1988). Paramecium. Berlin: Springer.
Greczek-Stachura, M., Potekhin, A., Przybos, E., Rautian, M., Skoblo, I., & Tarcz, S. (2012). Identification of Paramecium bursaria syngens through molecular markers—comparative analysis of three loci in the nuclear and mitochondrial DNA. Protist, 163(5), 671–685.
Guindon, S., & Gascuel, O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology, 52(5), 696–704.
Gustincich, S., Manfioletti, G., Del Sal, G., Schneider, C., & Carninci, P. (1991). A fast method for high-quality genomic DNA extraction from whole human blood. BioTechniques, 11(3), 298–300. 302.
Hall, M. S., & Katz, L. A. (2011). On the nature of species: insights from Paramecium and other ciliates. Genetica, 139(5), 677–684.
Jörger, K. M., & Schrödl, M. (2013). How to describe a cryptic species? Practical challenges of molecular taxonomy. Frontiers in Zoology, 10.
Kadereit, G., Piirainen, M., Lambinon, J., & Vanderpoorten, M. (2012). Cryptic taxa should have names: reflections in the glasswort genus Salicornia (Amaranthaceae). Taxon, 61(6), 1227–1239.
Kahl, A. (1935). Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria) 4. Peritricha und Chonotricha. (Vol. 30, Tierwelt Dtl.). Jena: G. Fischer.
Kosakyan, A., Heger, T. J., Leander, B. S., Todorov, M., Mitchell, E. A. D., & Lara, E. (2012). COI barcoding of nebelid testate amoebae (Amoebozoa: Arcellinida): extensive cryptic diversity and redefinition of the Hyalospheniidae Schultze. Protist, 163(3), 415–434.
Krenek, S., Petzoldt, T., & Berendonk, T. U. (2012). Coping with temperature at the warm edge—patterns of thermal adaptation in the microbial eukaryote Paramecium caudatum. PLoS ONE, 7(3), e30598.
Kreutz, M., Stoeck, T., & Foissner, W. (2012). Morphological and molecular characterization of Paramecium (Viridoparamecium nov. subgen.) chlorelligerum Kahl 1935 (Ciliophora). Journal of Eukaryotic Microbiology, 59(6), 548–563.
Kruskal, J. B., & Wish, M. (1978). Multidimensional scaling (Quantitative applications in the social sciences, vol. Nr. 11). Beverly Hills and London: SAGE Publications.
Leliaert, F., Verbruggen, H., Wysor, B., & De Clerck, O. (2009). DNA taxonomy in morphologically plastic taxa: algorithmic species delimitation in the Boodlea complex (Chlorophyta: Cladophorales). Molecular Phylogenetics and Evolution, 53(1), 122–133.
Medlin, L., Elwood, H. J., Stickel, S., & Sogin, M. L. (1988). The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene, 71(2), 491–499.
Messing, J. (1983). New M13 vectors for cloning. Methods in Enzymology, 101, 20–78.
Miller, M. A., Pfeiffer, W., & Schwartz, T. (2011). The CIPRES science gateway: A community resource for phylogenetic analyses. Paper presented at the Proceedings of the 2011 TeraGrid Conference: Extreme Digital Discovery, Salt Lake City, Utah.
Miyake, A. (1968). Induction of conjugation by chemical agents in Paramecium. Journal of Experimental Zoology, 167(3), 359.
Mohammed, A. H. H., & Nashed, N. N. (1968–1969). Paramecium wichtermani n. sp. with notes on other species of Paramecium common in fresh-water bodies in the area of Cairo and its environs. Zoological Society of Egypt Bulletin, (22), 89–104.
Müller, O. F. (1786). Animalcula infusoria fluviatilia et marina: Hauniae.
Murray, R. G., & Schleifer, K. H. (1994). Taxonomic notes: a proposal for recording the properties of putative taxa of procaryotes. International Journal of Systematic Bacteriology, 44(1), 174–176.
Murray, R. G., & Stackebrandt, E. (1995). Taxonomic note: implementation of the provisional status Candidatus for incompletely described procaryotes. International Journal of Systematic Bacteriology, 45(1), 186–187.
Pawlowski, J., Audic, S., Adl, S., Bass, D., Belbahri, L., Berney, C., et al. (2012). CBOL protist working group: barcoding eukaryotic richness beyond the animal, plant, and fungal kingdoms. PLoS Biology, 10(11), e1001419.
Pruesse, E., Peplies, J., & Glöckner, F. O. (2012). SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics, 28(14), 1823–1829.
Przybos, E., Tarcz, S., Potekhin, A., Rautian, M., & Prajer, M. (2012). A two-locus molecular characterization of Paramecium calkinsi. Protist, 163(2), 263–273.
Przybos, E., Rautian, M., Surmacz, M., & Bieliavskaya, A. (2013). New stands of species of the Paramecium aurelia complex (Ciliophora, Protista) in Russia (Siberia, Kamchatka). Folia Biologica-Krakow, 61(1–2), 41–45.
Przybos, E., Tarcz, S., Rautian, M., & Lebedeva, N. (2014). The first European stand of Paramecium sonneborni (P. aurelia complex), a species known only from North America (Texas, USA). European Journal of Protistology, 50(3), 236–247.
Powers, J. H., & Mitchell, C. (1910). A new species of Paramecium (P. multimicronucleata) experimentally determined. Biological Bulletin, 19:324–332.
Rambaut, A. (2012). FigTree v.1.4.0. http://tree.bio.ed.ac.uk/software/figtree/.
Ronquist, F., & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19(12), 1572–1574.
Sánchez, R., Serra, F., Tárraga, J., Medina, I., Carbonell, J., Pulido, L., et al. (2011). Phylemon 2.0: a suite of web-tools for molecular evolution, phylogenetics, phylogenomics and hypotheses testing. Nucleic Acids Research, 39(suppl 2), W470–W474.
Schrallhammer, M., Fokin, S. I., Schleifer, K. H., & Petroni, G. (2006). Molecular characterization of the obligate endosymbiont “Caedibacter macronucleorum” Fokin and Görtz, 1993 and of its host Paramecium duboscqui strain Ku4-8. Journal of Eukaryotic Microbiology, 53(6), 499–506.
Shi, X. B., Jin, M. L., & Liu, G. J. (1997). Rediscovery of Paramecium duboscqui Chatton & Brachon, 1933, and a description of its characteristics. Journal of Eukaryotic Microbiology, 44(2), 134–141.
Simon, E. M., Nanney, D. L., & Doerder, F. P. (2008). The “Tetrahymena pyriformis” complex of cryptic species. Biodiversity and Conservation, 17(2), 365–380.
Skaloud, P., & Rindi, F. (2013). Ecological differentiation of cryptic species within an asexual protist morphospecies: a case study of filamentous green alga Klebsormidium (Streptophyta). Journal of Eukaryotic Microbiology, 60(4), 350–362.
Skovorodkin, I. N. (1990). A device for immobilization of biological objects in the light microscope studies. Cytologia (St. Petersburg), 32, 515–519 (in Russian with English summary).
Sneath, P. H. A., & Sokal, R. R. (1973). Numerical taxonomy: the principles and practice of numerical classification. San Francisco: Freeman.
Sonneborn, T. M. (1975). The Paramecium aurelia complex of fourteen sibling species. Transactions of the American Microscopical Society, 94, 155–178.
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22(21), 2688–2690.
Stamatakis, A., Hoover, P., & Rougemont, J. (2008). A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology, 57(5), 758–771.
Stoeck, T., Welter, H., Seitz-Bender, D., Kusch, J., & Schmidt, H. J. (2000). ARDRA and RAPD-fingerprinting reject the sibling species concept for the ciliate Paramecium caudatum (Ciliophora, Protoctista). Zoologica Scripta, 29(1), 75–82.
Strüder-Kypke, M. C., & Lynn, D. H. (2010). Comparative analysis of the mitochondrial cytochrome c oxidase subunit I (COI) gene in ciliates (Alveolata, Ciliophora) and evaluation of its suitability as a biodiversity marker. Systematics and Biodiversity, 8(1), 131–148.
Strüder-Kypke, M. C., Wright, A. D. G., Fokin, S. I., & Lynn, D. H. (2000). Phylogenetic relationships of the genus Paramecium inferred from small subunit rRNA gene sequences. Molecular Phylogenetics and Evolution, 14(1), 122–130.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Molecular Biology and Evolution, 30(12), 2725–2729.
Tarcz, S., Potekhin, A., Rautian, M., & Przybos, E. (2012). Variation in ribosomal and mitochondrial DNA sequences demonstrates the existence of intraspecific groups in Paramecium multimicronucleatum (Ciliophora, Oligohymenophorea). Molecular Phylogenetics and Evolution, 63(2), 500–509.
Tarcz, S., Przybos, E., & Surmacz, M. (2013). An assessment of haplotype variation in ribosomal and mitochondrial DNA fragments suggests incomplete lineage sorting in some species of the Paramecium aurelia complex (Ciliophora, Protozoa). Molecular Phylogenetics and Evolution, 67(1), 255–265.
Tautz, D., Arctander, P., Minelli, A., Thomas, R. H., & Vogler, A. P. (2003). A plea for DNA taxonomy. Trends in Ecology & Evolution, 18(2), 70–74.
Thomas, R. C., Willette, D. A., Carpenter, K. E., & Santos, M. D. (2014). Hidden diversity in sardines: genetic and morphological evidence for cryptic species in the Goldstripe Sardinella, Sardinella gibbosa (Bleeker, 1849). PLoS ONE, 9(1).
Wenrich, D. H. (1928). Paramecium woodruffi nov. spec. (Ciliata). Transactions of the American Microscopical Society, 47, 256–261.
Wichterman, R. (1953). The biology of Paramecium. New York: Blakiston.
Wichterman, R. (1986). The biology of Paramecium (2nd ed.). New York: Plenum.
Woodruff, L. L. (1921). The structure, life history, and intrageneric relationships of Paramecium calkinsi, sp. nov. Biological Bulletin, 41, 171–180.
Woodruff, L. L., & Spencer, H. (1923). Paramecium polycaryum, sp. nov. Proceedings of the Society for Experimental Biology and Medicine, 20, 338–339.
Wylezich, C., Meisterfeld, R., Meisterfeld, S., & Schlegel, M. (2002). Phylogenetic analyses of small subunit ribosomal RNA coding regions reveal a monophyletic lineage of euglyphid testate amoebae (order Euglyphida). Journal of Eukaryotic Microbiology, 49(2), 108–118.
Yi, Z., Strueder-Kypke, M., Hu, X., Lin, X., & Song, W. (2014). Sampling strategies for improving tree accuracy and phylogenetic analyses: a case study in ciliate protists, with notes on the genus Paramecium. Molecular Phylogenetics and Evolution, 71, 142–148.
Zhao, Y., Gentekaki, E., Yi, Z., & Lin, X. (2013). Genetic differentiation of the mitochondrial cytochrome oxidase c subunit I gene in genus Paramecium (Protista, Ciliophora). PLoS ONE, 8(10), e77044.
Acknowledgments
This work was supported by a grant from the Italian Ministry of University and Research—MIUR (Ministerio Italiano dell’ Universita e della Ricerca) to S. I. Fokin and by the German Research Foundation (DFG, grant BE-2299/3-1,2,3 and BE 2299/5-1) to T. U. Berendonk, as well as by the European Commission FP7-PEOPLE-2009-IRSES project CINAR PATHOBACTER (247658) and by the BMBS COST Action BM1102. We are grateful to D. Ammermann for sampling in Porto Alegri, to Cora Baier and M. Horn for their help with 18S-rDNA sequence of “Eucandidatus P. brazilianum”, to M. Müller, who provided a possibility of our sampling at Balaton lake region and to Dana Barth for providing cells of P. buetschlii sp. nov. and DNA samples for molecular analyses. Further gratitude is expressed to two anonymous reviewers whose valuable comments allowed an essential improvement of the manuscript.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical standards
All the experiments in this study comply with the current laws of the country in which they were performed and do not infringe human and animal rights.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Krenek, S., Berendonk, T.U. & Fokin, S.I. New Paramecium (Ciliophora, Oligohymenophorea) congeners shape our view on its biodiversity. Org Divers Evol 15, 215–233 (2015). https://doi.org/10.1007/s13127-015-0207-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-015-0207-9