Abstract
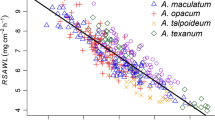
The debate surrounding Bergmann’s rule, in which the body size of animals is predicted to be larger in cooler environments, is still open concerning ectotherms. Our goal was to test this rule in the broadest ranging amphibian species Salamandrella keyserlingii. We determined age and body size in a cooler region (Darhadyn, Mongolia: mean yearly air temperature = –8.31 °C) using skeletochronology, and compared their differences in altitude, latitude, and temperature with those of a warmer area (Kushiro, Japan: 7.98 °C). In Darhadyn, both sexes reached sexual maturity at 5–6 years of age (growth coefficient: male = 0.585, female = 0.266), 2–3 years later than those in Kushiro (male = 1.341, female = 1.129). Mean body size was smaller in Darhadyn (53.08 mm) than in Kushiro (57.63 mm) for males despite their constant metamorphic size around 30 mm. We also analyzed data available from published studies for 27 populations within the geographic range of this species from 43 to 69°N across a 2,900-km long latitudinal gradient. The analysis indicated an intraspecific tendency to decrease body size with increased latitude from 43 to 57°N, to increase size from 57 to 69°N, and to decrease body size with decreased temperature from 8 to –7 °C and increase size from –7 to –15 °C. This pattern does not follow the intraspecific extension of Bergmann’s rule and may follow the converse of Terentjev’s optimum rule—a rule formulated to be an inverted-U shaped curve between increased latitude (or decreased temperature) and increased body size.




Similar content being viewed by others
References
Adams, D. C., & Church, J. O. (2008). Amphibians do not follow Bergmann’s rule. Evolution, 62, 413–420.
Allen, J. A. (1877). The influence of physical conditions in the genesis of species. Radical Review, 1, 108–140.
Amat, F., Oromí, N., & Sanuy, D. (2010). Body size, population size, and age structure of adult Palmate newts (Lissotriton helveticus) in Pyrenean lakes. Journal of Herpetology, 44, 313–319.
Anonymous. (2007). The times comprehensive atlas of the world, 12th edn. London: Times.
Arntzen, J. W. (2000). A growth curve for the newt Triturus cristatus. Journal of Herpetology, 34, 227–232.
Ashton, K. G. (2002). Do amphibians follow Bergmann’s rule? Canadian Journal of Zoology, 80, 708–716.
Ashton, K. G. (2004). Sensitivity of intraspecific latitudinal clines of body size for tetrapods to sampling, latitude and body size. Integrative and Comparative Biology, 44, 403–412.
Ashton, K. G., & Feldman, C. R. (2003). Bergmann’s rule in nonavian reptiles: turtles follow it and snakes reverse it. Evolution, 57, 1151–1163.
Bassarukin, A. M., & Borkin, L. J. (1984). Distribution, ecology, and morphological variability of the Siberian salamander, Hynobius keyserlingii, of the Sakhalin Island (in Russian with English summary). In L. J. Borkin (Ed.), Ecology and faunistics of amphibians and reptiles of the USSR and adjacent countries. Proceedings of the Zoological Institute, vol. 124 (pp. 12–54). Leningrad: USSR Academy of Sciences.
Bergmann, C. (1847). Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Göttinger Studien, 3, 595–708.
Berman, D. I., Derenko, M. V., Malyarchuk, B. A., Grzybowski, T., Kryukov, A. P., & Miscicka-Sliwka, D. (2005a). Genetic polymorphism in the Siberian salamander (Salamandrella keyserlingii, Caudata, Amphibia) in its geographic range and the cryptic species S. schrenckii from Primorye (in Russian). Doklady Akademii Nauk (= Proceedings of the Russian Academy of Sciences), 403, 427–429.
Berman, D. I., Derenko, M. V., Malyarchuk, B. A., Grzybowski, T., Kryukov, A. P., & Miscicka-Sliwka, D. (2005b). Intraspecific genetic differentiation of Siberian newt (Salamandrella keyserlingii, Amphibia, Caudata) and cryptic species S. schrenckii from the Russian South-East (in Russian with English summary). Zoologicheskii Zhurnal, 84, 1374–1388.
Berman, D. I., Derenko, M. V., Malyarchuk, B. A., Bulakhova, N. A., Grzybowski, T., Kryukov, A. P., & Leirikh, A. N. (2009). Range and genetic polymorphism of Salamandrella schrenckii (Amphibia, Caudata, Hynobiidae) (in Russian with English summary). Zoologicheskii Zhurnal, 88, 530–545.
Blackburn, T. M., Gaston, K. J., & Loder, N. (1999). Geographic gradients in body size: a clarification of Bergmann’s rule. Diversity and Distributions, 5, 165–174.
Borkin, L. J. (1994). Systematics. The Siberian newt (Salamandrella keyserlingii Dybowski, 1870) (in Russian). In E. I. Vorobyeva (Ed.), Zoogeography, systematics, and morphology (pp. 54–80). Moscow: Nauka.
Borkin, L. J. (1999). Salamandrella keyserlingii Dybowski, 1870. Sibirischer Winkelzahnmolch. In K. Grossenbacher & B. Thiesmeier (Eds.), Handbuch der Reptilien und Amphibien Europas, Urodela 1 (Vol. 4/1, pp. 21–55). Wiesbaden: Aula.
Borkin, L. J., Belimov, G. T., & Sedalishchev, V. T. (1984). New data on distribution of amphibians and reptiles in Yakutia (in Russian with English summary). In L. J. Borkin (Ed.), Ecology and faunistics of amphibians and reptiles of the USSR and adjacent countries. Proceedings of the Zoological Institute, vol. 124 (pp. 89–101). Leningrad: USSR Academy of Sciences.
Caetano, M. H., & Castanet, J. (1993). Variability and microevolutionary patterns in Triturus marmoratus from Portugal: age, size, longevity and individual growth. Amphibia-Reptilia, 14, 117–129.
Charnov, E. L. (1993). Life history invariants. Oxford: Oxford University Press.
Cogalniceanu, D., & Miaud, C. (2002). Age, survival and growth in Triturus dobrogicus (Amphibia, Urodela) from the lower Danube floodplain. International Association for Danube Research, 34, 777–783.
Cogalniceanu, D., & Miaud, C. (2003). Population age structure and growth of four syntopic amphibian species inhabiting a large river floodplain. Canadian Journal of Zoology, 81, 1096–1106.
Cvetkovic, D., Tomasevic, N., Ficetola, G. F., Crnobrnnja-Isailovic, J., & Miaud, C. (2009). Bergmann’s rule in amphibians: combining demographic and ecological parameters to explain body size variation among populations in the common toad Bufo bufo. Journal of Zoological Systematics and Evolutionary Research, 47, 171–180.
D’Agostino, R. B., Belanger, A., & D’Agostino, R. B., Jr. (1990). A suggestion for using powerful and informative tests for normality. The American Statistician, 44, 316–321.
Diaz-Paniagua, C., Mateo, J. A., & Andreu, A. C. (1996). Age and size structure of populations of small marbled newts (Triturus marmoratus pygmaeus) from Doñana National Park (SW Spain). A case of dwarfism among dwarfs. Journal of Zoology (London), 239, 83–92.
Dokuchaev, N. E., Andreev, A. V., & Atrashkevich, T. N. (1984). Materials on distribution and biology of Hynobius keyserlingii in the Far North-West of Asia (in Russian with English summary). In L. J. Borkin (Ed.), Ecology and faunistics of amphibians and reptiles of the USSR and adjacent countries. Proceedings of the Zoological Institute, vol. 124 (pp. 109–114). Leningrad: USSR Academy of Sciences.
Dunham, A. E. (1978). Food availability as a proximate factor influencing individual growth rates in the iguanid lizard Sceloporus merriami. Ecology, 59, 770–778.
Dybowski, B. (1870). Beitrag zur Kenntnis der Wassermolche Sibiriens. Verhandllungen der kaiserlich-königlichen zoologish-botanischen Gesellschaft in Wien, 20, 237–242.
Grigoriev, O. V., & Erdakov, L. N. (1981). Circadian activity of the Siberian salamander (Hynobius keyserlingii) at summer period (in Russian). In L. J. Borkin (Ed.), Herpetological investigations in Siberia and Far East (pp. 41–45). Leningrad: Zoological Institute, USSR Academy of Sciences.
Hasumi, M. (1996). Times required for ovulation, egg sac formation, and ventral gland secretion in the salamander Hynobius nigrescens. Herpetologica, 52, 605–611.
Hasumi, M. (2001). Secondary sexual characteristics of the salamander Salamandrella keyserlingii: throat coloration. Herpetological Review, 32, 223–225.
Hasumi, M. (2010). Age, body size, and sexual dimorphism in size and shape in Salamandrella keyserlingii (Caudata: Hynobiidae). Evolutionary Biology, 37, 38–48.
Hasumi, M., & Iwasawa, H. (1987a). Geographic variation in the tail of the Japanese salamander, Hynobius lichenatus, with special reference to taxonomic bearing. Zoological Science, 4, 159–166.
Hasumi, M., & Iwasawa, H. (1987b). Geographic variation in morphological characters of the Japanese salamander, Hynobius lichenatus. Science Reports of Niigata University, Series D (Biology), 24, 15–30.
Hasumi, M., & Kanda, F. (2007). Phenological activity estimated by movement patterns of the Siberian salamander near a fen. Herpetologica, 63, 163–175.
Hasumi, M., & Watanabe, Y. G. (2007). An efficient method for skeletochronology. Herpetological Review, 38, 404–406.
Hasumi, M., Hongorzul, T., Munkhchuluun, B., Taivanjargal, B., Hurelbaatar, E., Uuganbayar, A., Sano, T., & Nishida, H. (2007). Use of satellite imagery to find the salamander Salamandrella keyserlingii at Darhadyn Wetland, Mongolia. Herpetological Review, 38, 56–58.
Hasumi, M., Hongorzul, T., & Terbish, K. (2009). Burrow use by Salamandrella keyserlingii (Caudata: Hynobiidae). Copeia, 2009, 46–49.
Hasumi, M., Hongorzul, T., & Terbish, K. (2011). Animal species diversity at a land–water ecotone in Mongolia. Limnology, 12, 37–45.
Hawkins, B. A., & Diniz-Filho, J. A. (2004). ‘Latitude’ and geographic patterns in species richness. Ecography, 27, 268–272.
Hemelaar, A. (1988). Age, growth and other population characteristics of Bufo bufo from different latitudes and altitudes. Journal of Herpetology, 22, 369–388.
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G., & Javis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25, 1965–1978.
Ishchenko, V. G., & Berman, D. I. (1995). Population structure (in Russian). In E. I. Vorobyeva (Ed.), The Siberian newt (Salamandrella keyserlingii Dybowski, 1870). Ecology, behavior, and conservation (pp. 141–157). Moscow: Nauka.
Ishchenko, V. G., Ledenzov, A. V., Godina, L. B., & Kuzmin, S. L. (1995). Development and growth (in Russian). In E. I. Vorobyeva (Ed.), The Siberian newt (Salamandrella keyserlingii Dybowski, 1870). Ecology, behavior, and conservation (pp. 103–124). Moscow: Nauka.
Kashchenko, N. F. (1896). Salamandrella keyserlingii (in Russian).Izvestiya Tomskogo Universiteta, 10, 1–13.
King, M. B., & Queen, N. M. (1979). Use of rational functions for representing data. Journal of Chemical and Engineering Data, 24, 178–181.
Kostin, A. A. (1934). Amphibian fauna of North Manchuria and adjacent territory. I. Salamandrella keyserlingii Dybowski. Siberian four-toed newt. Systematic and biological outline. 1st part (in Russian). In Anonymous (Ed.), The annual of the club of natural science and geography of the YMCA, vol. 1 (for 1933) (pp. 160–182, 1 plate). Harbin: Club of Natural Science and Geography of the YMCA.
Kostin, A. A. (1942). Amphibian fauna of North Manchuria and adjacent territory. 3. Salamandrella keyserlingii Dybowski – Siberian four-fingered salamander (Systematic-biological outline. Part 2). In Anonymous (Ed.), Scientific works of the Przewalsky research association members (pp. 5–24). Harbin: Przewalsky Research Association.
Krizmanic, I., Vukov, T. D., & Kalezic, M. L. (2005). Bergmann’s rule is size-related in European newts (Triturus). Herpetological Journal, 15, 205–206.
Kusano, T., Ueda, T., & Nakagawa, H. (2006). Body size and age structure of breeding populations of the salamander, Hynobius tokyoensis (Caudata: Hynobiidae). Current Herpetology, 25, 71–78.
Kuzmin, S. L. (1994). The geographical range of Salamandrella keyserlingii: ecological and historical implications. Abhandlungen und Berichte für Naturkunde, 17, 177–183.
Larionov, P. D. (1976). Reproduction of Hynobius keyserlingii near Yakutsk (in Russian with English summary). Zoologicheskii Zhurnal, 55, 1259–1261.
Laugen, A. T., Laurila, A., Jönsson, K. I., Söderman, F., & Merilä, J. (2005). Do common frogs (Rana temporaria) follow Bergmann’s rule? Evolutionary Ecology Research, 7, 717–731.
Ledenzov, A. V. (1986). Materialien zur Lebensdauer und Wachstum vom sibirischen Winkelzahnmolch (Hynobius keyserlingii Dyb.) in der Mongolei (in Russian with separate German summary, p. 239). In E. I Vorobyeva (Ed.), Herpetologische Untersuchungen in der Mongolischen Volksrepublik, Moskau (pp. 73–77). Moscow: AN Severtsov Institute of Animal Evolutionary Morphology and Ecology, USSR Academy of Sciences.
Lee, J.-H., & Park, D. (2008). Effects of physical parameters and age on the order of entrance of Hynobius leechii to a breeding pond. Journal of Ecology and Field Biology, 31, 183–191.
Litvinchuk, S. N., & Borkin, L. J. (2009). Evolution, systematics, and distribution of crested newts (Triturus cristatus complex) in Russia and adjacent countries (in Russian). St Petersburg: Evropeisky Dom.
Litvinov, N. I., & Skuratov, N. V. (1986). The ecology of Siberian salamander in mountains of Hovsgol region (in Russian). In A. H. Filippov (Ed.), Natural condition and biological resources of Hovsgol region (pp. 131–134). Irkutsk: Irkutsk University Press.
Lovich, J. E., & Gibbons, J. W. (1992). A review of techniques for quantifying sexual size dimorphism. Growth, Development, and Aging, 56, 269–281.
Malyarchuk, B., Derenko, M., Berman, D., Perkova, M., Grzybowski, T., Lejrikh, A., & Bulakhova, N. (2010). Phylogeography and molecular adaptation of Siberian salamander Salamandrella keyserlingii based on mitochondrial DNA variation. Molecular Phylogenetics and Evolution, 56, 562–571.
Matsui, M., Yoshikawa, N., Tominaga, A., Sato, T., Takenaka, S., Tanabe, S., Nishikawa, K., & Nakabayashi, S. (2008). Phylogenetic relationships of two Salamandrella species as revealed by mitochondrial DNA and allozyme variation (Amphibia: Caudata: Hynobiidae). Molecular Phylogenetics and Evolution, 48, 84–93.
Mayr, E. (1965). Animal species and evolution. Cambridge: Harvard University Press.
Miaud, C., Guyetant, R., & Faber, H. (2000). Age, size, and growth of the Alpine newt, Triturus alpestris (Urodela: Salamandridae), at high altitude and a review of life-history trait variation throughout its range. Herpetologica, 56, 135–144.
Miaud, C., Andreone, F., Ribéron, A., De Michelis, S., Clima, V., Castanet, J., Francillon-Vieillot, H., & Guyétant, R. (2001). Variations in age, size at maturity and gestation duration among two neighbouring populations of the alpine salamander (Salamandra lanzai). Journal of Zoology (London), 254, 251–260.
Morrison, C., & Hero, J.-M. (2003). Geographic variation in life-history characteristics of amphibians: a review. Journal of Animal Ecology, 72, 270–279.
Munkhbayar, K. (1967). Hynobius keyserlingii (in Mongolian). BNMAU Shinjlekh Ukhaany Academijn Medee, 2, 26–31.
Munkhbayar, K. (1976). Mongolian amphibians and reptiles (in Mongolian). Ulaanbaatar: Publishing House.
Obst, F. J. (1963). Amphibien und Reptilien aus dem Mongolei. Mitteilungen aus dem Zoologischen Museum in Berlin, 39, 361–370.
Olalla-Tárraga, M. Á., & Rodríguez, M. Á. (2007). Energy and interspecific body size patterns of amphibian faunas in Europe and North America: anurans follow Bergmann’s rule, urodeles its converse. Global Ecology and Biogeography, 16, 606–617.
Olalla-Tárraga, M. Á., Rodríguez, M. Á., & Hawkins, A. (2006). Broad-scale patterns of body size in squamate reptiles of Europe and North America. Journal of Biogeography, 33, 781–793.
Olalla-Tárraga, M. Á., Bini, L. M., Diniz-Filho, J. A. F., & Rodríguez, M. Á. (2010). Cross-species and assemblage-based approaches to Bergmann’s rule and the biogeography of body size in Plethodon salamanders of eastern North America. Ecography, 33, 362–368.
Olgun, K., Miaud, C., & Gautier, P. (2001). Age, growth, and survivorship in the viviparous salamander Mertensiella luschani from southwestern Turkey. Canadian Journal of Zoology, 79, 1559–1567.
Palo, J. U., O’Hara, R. B., Laugen, A. T., Laurila, A., Primmer, C. R., & Merilä, J. (2003). Latitudinal divergence of common frog (Rana temporaria) life history traits by natural selection: evidence from a comparison of molecular and quantitative genetic data. Molecular Ecology, 12, 1963–1978.
Pavlov, P. A. (1934). Données pour servir à létude de la faune de la Chine du nord, de la Mandchourie et de Mongolie. Amphibiens Caudata, Apoda et Costata. Publications des Musée Hoang ho Pai ho Tien Tsin, 32, 1–32, 4 plates.
Ray, C. (1960). The application of Bergmann’s rule and Allen’s rule to the poikilotherms. Journal of Morphology, 106, 85–109.
Schäuble, C. S. (2004). Variation in body size and sexual dimorphism across geographical and environmental space in the frogs Limnodynastes tasmaniensis and L. peronii. Biological Journal of the Linnean Society, 82, 39–56.
Shchepina, N. A., Borisova, N. G., Baldanova, D. P., & Rudneva, L. V. (2009). Amphibians of Buryatia (in Russian). Ulan-Ude: Buryatian Scientific Center, Siberian Division, Russian Academy of Sciences.
Shkatulova, A. P., Karasev, G. L., & Khundanov, L. E. (1978). Amphibians and reptiles of Transbaikalia (Buryat ASSR and Chita region) (in Russian). Ulan-Ude: Buryatskoe Knizhnoe.
Shurygina, K. I. (1969). Toward the biology of the Siberian salamander of Sakhalin Island (in Russian). Voprosy Biologii Tula, 2, 154–162.
Smirina, E. M. (1994). Age determination and longevity in amphibians. Gerontology, 40, 133–146.
Stamps, J. A., & Andrews, R. M. (1992). Estimating asymptotic size using the largest individuals per sample. Oecologia, 92, 503–512.
Stamps, J. A., Krishnan, V. V., & Andrews, R. M. (1994). Analyses of sexual size dimorphism using null growth-based models. Copeia, 1994, 598–613.
Stillwell, R. C. (2010). Are latitudinal clines in body size adaptive? Oikos, 119, 1387–1390.
Storey, K. B., & Storey, J. M. (1992). Natural freeze tolerance in ectothermic vertebrates. Annual Review of Physiology, 54, 619–637.
Tagirova, V. T. (1979). Biological peculiarities of Hynobius keyserlingii in Priamurie (in Russian). In A. P. Nechaev (Ed.), Okhrana i Ratsionalnoe Ispolzovanie Flory i Fauny Nizhnego Priamuriya i Sakhalina (= Protection and rational management of flora and fauna of lower Amur River area and Sakhalin) (pp. 122–130). Khabarovsk: Khabarovsk State Pedagogical Institute.
Tarling, G. A., & Cuzin-Roudy, J. (2003). Synchronization in the molting and spawning activity of northern krill (Meganyctiphanes norvegica) and its effect on recruitment. Limnology and Oceanography, 48, 2020–2033.
Terentjev, P. V. (1946). An attempt of application of mathematic statistics to zoogeography (in Russian). Vestnik Leningrad University, (2), 105–110.
Terentjev, P. V. (1947). On the applicability of Bergmann’s rule to animals with stable body temperature (in Russian). Vestnik Leningrad University, (12), 41–46.
Terentjev, P. V. (1951). Influence of climatic temperature on size of snakes and anurans (in Russian). Bulletin of the Moscow Society for Naturalists, Biology, 56(2), 14–23.
Terentjev, P. V. (1966). Methodical considerations on study of intraspecific geographic variation (in Russian). In S. S. Shvarts (Ed.), Intraspecific geographic variation in terrestrial vertebrates and microevolution (pp. 3–20). Sverdlovsk: Institute of Biology, Ural Branch, USSR Academy of Sciences.
Thomas, G. H. (2009). Bergmann’s idiosyncratic rule: a role for fecundity selection? Molecular Ecology, 18, 1027–1029.
Ushakov, V. A. (1978). New data on distribution of Hynobius keyserlingii (Hynobiidae) in the European part of the USSR (in Russian with English summary). Zoologicheskii Zhurnal, 57, 799–801.
Vershinin, V. L. (2007). Amphibians and reptiles of Ural (in Russian). Ekaterinburg: Ural Division, Russian Academy of Sciences.
von Bertalanffy, L. (1938). A quantitative theory of organic growth (inquiries on growth laws. II). Human Biology, 10, 181–213.
Voronov, G. A., Shurakov, A. I., & Kamensky, Y. I. (1971). On the biology of Hynobius keyserlingii in the Perm region (in Russian). Voprosy Ekologii i Teriologii (= Problems of Ecology and Theriology), 55, 70–74.
Wagner, A., Schabetsberger, R., Sztatecsny, M., & Kaiser, R. (2011). Skeletochronology of phalanges underestimates the true age of long-lived Alpine newts (Ichthyosaura alpestris). Herpetological Journal, 21, 145–148.
Walters, R. J., & Hassall, M. (2006). The temperature–size rule in ectotherms: may a general explanation exist after all? The American Naturalist, 167, 510–523.
Wise, S. E., & Buchanan, B. W. (1992). An efficient method for measuring salamanders. Herpetological Review, 23, 56–57.
Zeleznik, F. J. (1968). Quasi-Newton methods for nonlinear equations. Journal of the Association for Computing Machinery, 15, 265–271.
Acknowledgments
Cordial thanks are due to T. Hongorzul, E. Hurelbaatar, B. Taivanjargal, and A. Uuganbayar for their field assistance, T. Kusano for giving us information about literature, H. Ota for his advice on species names, and A. V. Andreev, A. M. Bassarukin, D. I. Berman, O. V. Grigoriev, V. G. Ishchenko, N. G. Ostashko, N. A. Shchepina, and V. L. Vershinin for permitting us to use their unpublished data. We are indebted to D. M. Sever and C. Angelini for critically reviewing the manuscript. We express our gratitude for the constructive comments of two anonymous reviewers and the proofreading and editing of the final version of the manuscript by T. A. G. Rittenhouse. This study was supported financially in part by Grants-in-Aid for Scientific Research from the Japan Fund for Global Environment (Environmental Restoration and Conservation Agency of Japan) (M. H.), the Aeon Environment Foundation (Japan) (M. H.), and the Russian Foundation of Basic Research (RFBR.08-04-01184) (L. B.).
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Appendix 2
Rights and permissions
About this article
Cite this article
Hasumi, M., Borkin, L.J. Age and body size of Salamandrella keyserlingii (Caudata: Hynobiidae): a difference in altitudes, latitudes, and temperatures. Org Divers Evol 12, 167–181 (2012). https://doi.org/10.1007/s13127-012-0091-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-012-0091-5