Abstract
Aim-Background
Totally implantable central venous access devices (TIVADs) are commonly used in patients to administer chemotherapy or long-term parenteral nutrition. In the present article, we report our experience with TIVADs.
Patients-Methods
In the period between 1 January 2009 and 31 December 2011, a total of 64 patients (18 men, 46 women) with an average age of 57 years (41–79 years) underwent implantation of a central vein catheter in an outpatient or inpatient setting. The indications were chemotherapy (n=60) and total parenteral nutrition (n=4).
Results
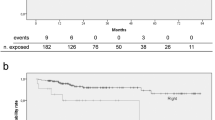
The cancer patients had been operated on for cancer of the colon (n=26), cancer of breast (n=23), stomach cancer (n=8) and haematologic malignancies (n=3). The other 4 patients had small intestine syndrome after extensive resection of the small intestine for mesenteric ischaemia. The median follow-up duration was 8.8 months (range 0.2–33 months). All intraports were inserted via the subclavian vein by the same surgical team using the aseptic percutaneous access technique. The complications included thrombosis in two cases (3.1%), followed by infection of the tip in one case (1.6%). No other complications or deaths were reported.
Conclusions
We confirm that the use of subcutaneous ports in adults with cancer or malnutrition is feasible for the majority of patients with low morbidity and absence of mortality. To avoid complications, the surgery team needs to be very experienced in regard to the positioning of the catheters. Furthermore, daily management of the catheters is of paramount importance.
Similar content being viewed by others
References
Broviac JW, Cole JJ, Scriber BHA. Silicone rubber atrial catheter for prolonged parenteral alimentation. Surg Gynecol Obstet 1973; 136:602–606.
Hickman RO, Buckner CD, Clift RA, et al. A modified right atrial catheter for access to the venous system in marrow transplant recipients. Surg Gynecol Obstet 1979; 148:871–875.
Niederhuber JE, Ensminger W, Gyves JW, et al. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery 1982;92:706–712.
Biffi R, De Braud F, Orsi F, Pozzi S, Arnaldi P, Goldhirsch A, Rotmensz N, Robertson C, Bellomi M, Andreoni B. A randomized, prospective trial of central venous ports connected to standard open-ended or Groshong catheters in adult oncology patients. Cancer 2001; 92(5):1204–1212.
Sitges-Serra A, Linares J, Garau J. Catheter sepsis: the clue is the hub. Surgery 1985; 97: 355–357.
Decker MD, Edwards KM. Central venous catheter infections. Pediatr Clin North Am 1988; 35: 579–612.
Van HoffJ, Berg AT, Seashore JH. The effect of right atrial catheters on infectious complications of chemotherapy. J Clin Oncol 1990; 8: 1255–1262.
Bothe A Jr, Piccione W, Ambrosino JJ, Benotti PN, Lokich JJ. Implantable central venous access system. Am J Surg 1984;147:565–569.
Kawasaki R, Morita S, Hisa N, Tsuji A, Noda Y. [Evaluation of 389 cases with a central venous access device inserted peripherally in the forearm] Gan To Kagaku Ryoho 1999;26(13):2055–2060.
Harvey WH, Pick TE, Reed K, Solenberger RI. A prospective evaluation of the Port-A-Cath implantable venous access system in chronically ill adults and children. Surg Gynecol Obstet 1989;169:495–500.
Couban S, Goodyear M, Burnell M, Dolan S, Wasi P, Barnes D, Macleod D, Burton E, Andreou P, Anderson DR. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J Clin Oncol 2005;23:4063–4069.
Boraks P, Seale J, Price J, Bass G, Ethell M, Keeling D, Mahendra P, Baglin T, Marcus R. Prevention of central venous catheter associated thrombosis using minidose warfarin in patients with haematological malignancies. Br J Haematol 1998;101(3):483–486.
Heaton DC, Han DY, Inder A. Minidose (1 mg) warfarin as prophylaxis for central vein catheter thrombosis. Intern Med J 2002;32(3):84–88.
Monreal M, Alastrue A, Rull M, Mira X, Muxart J, Rosell R, Abad A. Upper extremity deep venous thrombosis in cancer patients with venous access devices — prophylaxis with a low molecular weight heparin (Fragmin) Thromb Haemost 1996;75(2):251–253.
Reichardt P, Kretzschmar A, Biakhov M, Irwin D, Slabber C, Miller L, Bowden C. A phase III randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of daily low-molecular-weight heparin (dalteparin sodium, fragmin) in preventing catheter-related complications (CRCs) in cancer patients with central venous catheters (CVCs) Proc Am Soc Clin Oncol 2002;21:Abstr 1474.
Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol 2003;21:3665–3675.
Messing B, Peitra-Cohen S, Debure A, et al. Antibiotic-lock technique: a new approach to optimal therapy for catheter-related sepsis in home-parenteral nutrition patients. J Parent Ent Nutr 1988; 12: 185–188.
Barrios CH, Zuke JE, Blaes B, Hirsch JD, Lyss AP. Evaluation of an implantable venous access system in a general oncology population. Oncology 1992;49(6):474–478.
Elandt-Johnson RC, Johnson NL. Survival models and data analysis. New York: Wiley, 1980: 150–180.
Vescia S, Baumgärtner AK, Jacobs VR, Kiechle-Bahat M, Rody A, Loibl S, Harbeck N. Management of venous port systems in oncology: a review of current evidence. Ann Oncol 2008;19:9–15.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kouzanidis, N., Paraskevopoulos, J.A., Papazacharias, C. et al. Totally implantable venous access devices in surgical patients: Our 3-year experience. Hellenic J Surg 85, 180–184 (2013). https://doi.org/10.1007/s13126-013-0033-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13126-013-0033-5